Nebido 1000Mg/4Ml Solution For Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Nebido 1000 mg / 4ml, solution for injection.T
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml solution for injection contains 250 mg testosterone undecanoate corresponding to 157.9 mg testosterone.
Each ampoule / vial with 4 ml solution for injection contains 1000 mg testosterone undecanoate corresponding to 631.5 mg testosterone.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Solution for injection.
Clear, yellowish oily solution.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Testosterone replacement therapy for male hypogonadism when testosterone deficiency has been confirmed by clinical features and biochemical tests (see section 4.4).
4.2 Posology and method of administration Posology
One ampoule / vial of Nebido (corresponding to 1000 mg testosterone undecanoate) is injected every 10 to 14 weeks. Injections with this frequency are capable of maintaining sufficient testosterone levels and do not lead to accumulation.
Start of treatment
Serum testosterone levels should be measured before start and during initiation of treatment. Depending on serum testosterone levels and clinical symptoms, the first injection interval may be reduced to a minimum of 6 weeks as compared to the recommended range of 10 to 14 weeks for maintenance. With this loading dose, sufficient steady state testosterone levels may be achieved more rapidly.
Maintenance and individualisation of treatment
The injection interval should be within the recommended range of 10 to 14 weeks. Careful monitoring of serum testosterone levels is required during maintenance of treatment. It is advisable to measure testosterone serum levels regularly. Measurements should be performed at the end of an injection interval and clinical symptoms considered. These serum levels should be within the lower third of the normal range. Serum levels below normal range would indicate the need for a shorter injection interval. In case of high serum levels an extension of the injection interval may be considered.
Special populations
Paediatric population
Nebido is not indicated for use in children and adolescents and it has not been clinically evaluated in males under 18 years of age (see section 4.4).
Geriatric patients
Limited data do not suggest the need for a dosage adjustment in elderly patients (see section 4.4).
Patients with hepatic impairment
No formal studies have been performed in patients with hepatic impairment. The use of Nebido is contraindicated in men with past or present liver tumours (see section 4.3).
Patients with renal impairment
No formal studies have been performed in patients with renal impairment.
Method of administration
For intramuscular use.
The injections must be administered very slowly (over two minutes). Nebido is strictly for intramuscular injection. Care should be taken to inject Nebido deeply into the gluteal muscle following the usual precautions for intramuscular administration. Special care must be taken to avoid intravasal injection (see section 4.4 under “Application”). The contents of an ampoule / vial are to be injected intramuscularly immediately after opening. (For the ampoule see section 6.6 for instructions on opening the ampoule safely).
4.3 Contraindications
The use of Nebido is contraindicated in men with:
• androgen-dependent carcinoma of the prostate or of the male
mammary gland
• past or present liver tumours
• hypersensitivity to the active substance or to any of the excipients.
The use of Nebido in women is contraindicated.
4.4 Special warnings and precautions for use
Nebido is not recommended for use in children and adolescents.
Nebido should be used only if hypogonadism (hyper- and hypogonadotrophic) has been demonstrated and if other aetiology, responsible for the symptoms, has been excluded before treatment is started. Testosterone insufficiency should be clearly demonstrated by clinical features (regression of secondary sexual characteristics, change in body composition, asthenia, reduced libido, erectile dysfunction etc.) and confirmed by two separate blood testosterone measurements.
Elderly population
There is limited experience on the safety and efficacy of the use of Nebido in patients over 65 years of age. Currently, there is no consensus about age specific testosterone reference values. However, it should be taken into account that physiologically testosterone serum levels are lower with increasing age.
Medical examination and laboratory tests
Medical examinations
Prior to testosterone initiation, all patients must undergo a detailed examination in order to exclude a risk of pre-existing prostatic cancer. Careful and regular monitoring of the prostate gland and breast must be performed in accordance with recommended methods (digital rectal examination and estimation of serum PSA) in patients receiving testosterone therapy at least once yearly and twice yearly in elderly patients and at risk patients (those with clinical or familial factors). Local guidelines for safety monitoring under testosterone replacement therapy should be taken into consideration.
Laboratory tests
Testosterone level should be monitored at baseline and at regular intervals during treatment. Clinicians should adjust the dosage individually to ensure maintenance of eugonadal testosterone levels. In patients receiving long-term androgen therapy, the following laboratory parameters should also be monitored regularly: haemoglobin and haematocrit, liver function tests and lipid profile (see section 4.8).
Due to variability in laboratory values, all measures of testosterone should be carried out in the same laboratory.
Tumours
Androgens may accelerate the progression of sub-clinical prostatic cancer and benign prostatic hyperplasia.
Nebido should be used with caution in cancer patients at risk of hypercalcaemia (and associated hypercalciuria), due to bone metastases. Regular monitoring of serum calcium concentrations is recommended in these patients.
Cases of benign and malignant liver tumours have been reported in users of hormonal
substances such as androgen compounds. If severe upper abdominal complaints, liver enlargement or signs of intra-abdominal haemorrhage occur in men using Nebido, a liver tumour should be included in the differential-diagnostic considerations.
Cardiac, hepatic or renal insufficiency
In patients suffering from severe cardiac, hepatic or renal insufficiency or ischaemic heart disease, treatment with testosterone may cause severe complications characterised by oedema with or without congestive cardiac failure. In such case, treatment must be stopped immediately.
Hepatic or renal insufficiency
There are no studies undertaken to demonstrate the efficacy and safety of this medicinal product in patients with renal or hepatic impairment. Therefore, testosterone replacement therapy should be used with caution in these patients.
Cardiac insufficiency
Caution should be exercised in patients predisposed to oedema, e.g. in case of severe cardiac, hepatic, or renal insufficiency or ischaemic heart disease, as treatment with androgens may result in increased retention of sodium and water. In case of severe complications characterized by oedema with or without congestive heart failure treatment must be stopped immediately (see section 4.8).
Testosterone may cause a rise in blood pressure and Nebido should be used with caution in men with hypertension. As a general rule, the limitations of using intramuscular injections in patients with acquired or inherited blood clotting irregularities always have to be observed.
Other conditions
Nebido should be used with caution in patients with epilepsy and migraine, as the conditions may be aggravated.
Improved insulin sensitivity may occur in patients treated with androgens who achieve normal testosterone plasma concentrations following replacement therapy.
Certain clinical signs: irritability, nervousness, weight gain, prolonged or frequent erections may indicate excessive androgen exposure requiring dosage adjustment.
Pre-existing sleep apnoea may be potentiated.
Athletes treated for testosterone replacement in primary and secondary male hypogonadism should be advised that the medicinal product contains an active substance which may produce a positive reaction in anti-doping tests.
Androgens are not suitable for enhancing muscular development in healthy individuals or for increasing physical ability.
Nebido should be permanently withdrawn if symptoms of excessive androgen exposure persist or reappear during treatment with the recommended dosage regimen.
Application
As with all oily solutions, Nebido must be injected strictly intramuscularly and very slowly (over two minutes). Pulmonary microembolism of oily solutions can in rare cases lead to signs and symptoms such as cough, dyspnoea, malaise, hyperhidrosis, chest pain, dizziness, paraesthesia, or syncope. These reactions may occur during or immediately after the injection and are reversible. The patient should therefore be observed during and immediately after each injection in order to allow for early recognition of possible signs and symptoms of pulmonary oily microembolism. Treatment is usually supportive, e.g. by administration of supplemental oxygen.
Suspected anaphylactic reactions after Nebido injection have been reported.
4.5 Interaction with other medicinal products and other forms of interaction
Oral anti-coagulants
Testosterone and derivatives have been reported to increase the activity of coumarin derived oral anti-coagulants.-Patients receiving oral anti-coagulants require close monitoring, especially at the beginning or end of androgen therapy. Increased monitoring of the prothrombin time, and INR determinations, are recommended.
Other interactions
The concurrent administration of testosterone with ACTH or corticosteroids may enhance oedema formation; thus these active substances should be administered cautiously, particularly in patients with cardiac or hepatic disease or in patients predisposed to oedema.
Laboratory test interactions: Androgens may decrease levels of thyroxin-binding globulin resulting in decreased total T4 serum levels and increased resin uptake
of T3 and T4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
4.6 Fertility, pregnancy and lactation
Fertility
Testosterone replacement therapy may reversibly reduce spermatogenesis (see sections 4.8 and 5.3).
Pregnancy and breastfeeding
Nebido is not indicated for use in women and must not be used in pregnant or breast-feeding women (see section 4.3).
4.7 Effects on ability to drive and use machines
Nebido has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Summary of the safety profile
Regarding undesirable effects associated with the use of androgens, please also refer to section 4.4.
The most frequently reported undesirable effects during treatment with Nebido are acne and injection site pain.
Pulmonary microembolism of oily solutions can in rare cases lead to signs and symptoms such as cough, dyspnoea, malaise, hyperhidrosis, chest pain, dizziness, paraesthesia, or syncope. These reactions may occur during or immediately after the injection and are reversible. Cases suspected by the company or the reporter to represent oily pulmonary microembolism have been reported rarely in clinical trials (in > 1/10,000 and < 1/1,000 injections) as well as from postmarketing experience (see section 4.4).
Suspected anaphylactic reactions after Nebido injection have been reported.
Androgens may accelerate the progression of sub-clinical prostatic cancer and benign prostatic hyperplasia.
Table 1 below reports adverse drug reactions (ADRs) by MedDRA system organ classes (MedDRA SOCs) reported with Nebido. The frequencies are based on clinical trial data and defined as common (> 1/100 to < 1/10), uncommon (> 1/1000 to <1/100) and rare (> 1/10,000 to < 1/1,000). The ADRs were recorded in 6 clinical studies (N=422) and considered at least possibly causally related to Nebido.
Tabulated list of adverse reactions
Table 1: Categorised relative frequency of men with ADRs, by MedDRA SOC - based on pooled data of six, clinical trials, N=422 (100.0%), i.e.N=302 hypogonadal men treated with i.m. injections of 4 ml andN=120 with 3ml of TU250 mg/ml_
|
System Organ Class |
Common (> 1/100 to < 1/10) |
Uncommon (> 1/1000 to <1/100) |
Rare (> 1/10,000 to < 1/1,000) |
|
Blood and lymphatic system disorders |
Polycythaemia Haematocrit increased* Red blood cell count increased* Haemoglobin increased* | ||
|
Immune system disorders |
Hypersensitivity | ||
|
Metabolism and nutrition disorders |
Weight increased |
Increased appetite Glycosylated haemoglobin increased Hypercholesterolaemia Blood triglycerides increased Blood cholesterol increased | |
|
Psychiatric disorders |
Depression Emotional disorder Insomnia Restlessness Aggression Irritability | ||
|
Nervous system disorders |
Headache Migraine Tremor | ||
|
Vascular disorders |
Hot flush |
Cardiovascular disorder Hypertension Dizziness | |
|
Respiratory, thoracic and mediastinal disorders |
Bronchitis Sinusitis Cough Dyspnoea Snoring Dysphonia | ||
|
Gastrointestinal disorders |
Diarrhoea Nausea |
|
System Organ Class |
Common (> 1/100 to < 1/10) |
Uncommon (> 1/1000 to <1/100) |
Rare (> 1/10,000 to < 1/1,000) |
|
Hepatobiliary disorders |
Liver function test abnormal Aspartate aminotransferase increased | ||
|
Skin and subcutaneous tissue disorders |
Acne |
Alopecia Erythema Rash1 Pruritus Dry skin | |
|
Musculoskeletal and connective tissue disorders |
Arthralgia Pain in extremity Muscle disorders2 Musculoskeletal stiffness Blood creatine phosphokinase increased | ||
|
Renal and urinary disorders |
Urine flow decreased Urinary retention Urinary tract disorder Nocturia Dysuria | ||
|
Reproductive system and breast disorders |
Prostate specific antigen increased Prostate examination abnormal Benign prostate hyperplasia |
Prostatic intraepithelial neoplasia Prostate induration Prostatitis Prostatic disorder Libido changes Testicular pain Breast induration Breast pain Gynaecomastia Oestradiol increased Testosterone increased | |
|
General disorders and administration site conditions |
Various kinds of injection site reactions3 |
Fatigue Asthenia Hyperhidrosis4 | |
|
Injury, poisoning and procedural complications |
Pulmonary oil microembolism** |
*Respective frequency has been observed in relation to the use in testosterone containing products.
** Frequency is based on the number of injections.
The most appropriate MedDRA term to describe a certain adverse reaction is listed. Synonyms or related conditions are not listed, but should be taken into account as well.
Rash including Rash papular
4
Muscle disorders: Muscle spasm, Muscle strain and Myalgia Various kinds of injection site reaction: Injection site pain, Injection site discomfort, Injection site pruritus, Injection site erythema, Injection site haematoma, Injection site irritation, Injection site reaction Hyperhidrosis: Hyperhidrosis and Night sweats
Description of selected adverse reactions
Pulmonary microembolism of oily solutions can in rare cases lead to signs and symptoms such as cough, dyspnoea, malaise, hyperhidrosis, chest pain, dizziness, paraesthesia, or syncope. These reactions may occur during or immediately after the injections and are reversible. Cases suspected by the company or the reporter to represent oily pulmonary microembolism have been reported rarely in clinical trials (in > 1/10,000 and < 1/1,000 injections) as well as from postmarketing experience (see section 4.4).
In addition to the above mentioned adverse reactions, nervousness, hostility, sleep apnoea, various skin reactions including seborrhoea, increased hair growth, increased frequency of erections and in very rare cases jaundice have been reported under treatment with testosterone containing preparations.
Therapy with high doses of testosterone preparations commonly reversibly interrupts or reduces spermatogenesis, thereby reducing the size of the testicles; testosterone replacement therapy of hypogonadism can in rare cases cause persistent, painful erections (priapism). High-dosed or long-term administration of testosterone occasionally increases the occurrences of water retention and oedema.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No special therapeutic measure apart from termination of therapy with the medicinal product or dose reduction is necessary after overdose.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Androgens, 3-oxoandrosten (4) derivatives ATC code: G03B A03
Testosterone undecanoate is an ester of the naturally occurring androgen, testosterone. The active form, testosterone, is formed by cleavage of the side chain.
Testosterone is the most important androgen of the male, mainly synthesised in the testicles, and to a small extent in the adrenal cortex.
Testosterone is responsible for the expression of masculine characteristics during foetal, early childhood, and pubertal development and thereafter for maintaining the masculine phenotype and androgen-dependent functions (e.g. spermatogenesis, accessory sexual glands). It also performs functions, e.g. in the skin, muscles, skeleton, kidney, liver, bone marrow, and CNS.
Dependent on the target organ, the spectrum of activities of testosterone is mainly androgenic (e.g. prostate, seminal vesicles, epididymis) or protein-anabolic (muscle, bone, haematopoiesis, kidney, liver).
The effects of testosterone in some organs arise after peripheral conversion of testosterone to estradiol, which then binds to estrogen receptors in the target cell nucleus e.g. the pituitary, fat, brain, bone, and testicular Leydig cells.
5.2 Pharmacokinetic properties
- Absorption
Nebido is an intramuscularly administered depot preparation of testosterone undecanoate and thus circumvents the first-pass effect. Following intramuscular injection of testosterone undecanoate as an oily solution, the compound is gradually released from the depot and is almost completely cleaved by serum esterases into testosterone and undecanoic acid. An increase in serum levels of testosterone above basal values may be seen one day after administration.
Steady-state conditions
After the 1st intramuscular injection of 1000 mg testosterone undecanoate to hypogonadal men, mean Cmax values of 38 nmol/L (11 ng/mL) were obtained after 7 days. The second dose was administered 6 weeks after the 1st injection and maximum testosterone concentrations of about 50 nmol/L (15 ng/mL) were reached. A constant dosing interval of 10 weeks was maintained during the following 3 administrations and steady-state conditions were achieved between the 3rd and the 5th administration. Mean Cmax and Cmin values of testosterone at steady-state were about 37 (11 ng/mL) and 16 nmol/L (5 ng/mL), respectively. The median intra- and interindividual variability (coefficient of variation, %) of Cmin values was 22 % (range: 928%) and 34% (range: 25-48%), respectively.
- Distribution
In serum of men, about 98% of the circulating testosterone is bound to sex hormone binding globulin (SHBG) and albumin. Only the free fraction of testosterone is considered as biologically active. Following intravenous infusion of testosterone to elderly men, the elimination half-life of testosterone was approximately one hour and an apparent volume of distribution of about 1.0 l/kg was determined.
- Metabolism
Testosterone which is generated by ester cleavage from testosterone undecanoate is metabolised and excreted the same way as endogenous testosterone. The undecanoic acid is metabolised by B-oxidation in the same way as other aliphatic carboxylic acids. The major active metabolites of testosterone are oestradiol and dihydrotestosterone.
- Elimination
Testosterone undergoes extensive hepatic and extrahepatic metabolism. After the administration of radio-labelled testosterone, about 90% of the radioactivity appears in the urine as glucuronic and sulphuric acid conjugates and 6% appears in the faeces after undergoing enterohepatic circulation. Urinary medicinal products include androsterone and etiocholanolone. Following intramuscular administration of this depot formulation the release rate is characterised by a half life of 90±40 days.
5.3 Preclinical safety data
Toxicological studies have not revealed other effects than those which can be explained based on the hormone profile of Nebido.
Testosterone has been found to be non-mutagenic in vitro using the reverse mutation model (Ames test) or hamster ovary cells. A relationship between androgen treatment and certain cancers has been found in studies on laboratory animals. Experimental data in rats have shown increased incidences of prostate cancer after treatment with testosterone.
Sex hormones are known to facilitate the development of certain tumours induced by known carcinogenic agents. The clinical relevance of the latter observation is not known.
Fertility studies in rodents and primates have shown that treatment with testosterone can impair fertility by suppressing spermatogenesis in a dose dependent manner.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Benzyl benzoate Castor oil, refined
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
5 years
The medicinal product must be used immediately after first opening.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
Ampoule
5ml brown glass (type I) ampoules, containing a fill volume of 4 ml Pack size: 1 x 4 ml
Vial
6ml brown glass (type I ) vial with gray bromobutyl (foil-clad ETFE) injection stopper and bordered cap, containing a fill volume of 4 ml Pack size: 1 x 4 ml
6.6 Special precautions for disposal and other handling
The solution for intramuscular injection is to be visually inspected prior to use and only clear solutions free from particles should be used.
The medicinal product is for single use only and any unused solution should be discarded in accordance with local requirements.
Ampoule
Notes on handling the OPC (One-Point-Cut) ampoule:
There is a pre-scored mark beneath the coloured point on the ampoule eliminating the need to file the neck. Prior to opening, ensure that any solution in the upper part of the ampoule flows down to the lower part. Use both hands to open; while holding the lower part of the ampoule in one hand, use the other hand to break off the upper part of the ampoule in the direction away from the coloured point.
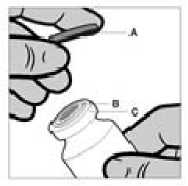
The vial is for single use only. The content of a vial is to be injected intramuscularly immediately after drawing up into the syringe. After removal of the plastic cap (A) do not remove the metal ring (B) or the crimp cap (C).
7. MARKETING AUTHORISATION HOLDER
Bayer plc Bayer House Strawberry Hill Newbury
Berkshire RG14 1JA United Kingdom
8 MARKETING AUTHORISATION NUMBER
PL 00010/0549
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 20th October 2004 Date of lastest renewal: 25th November 2008
10 DATE OF REVISION OF THE TEXT
23/03/2016