Phenylephrine 50 Micrograms/Ml Solution For Injection In Pre-Filled Syringe
The pre-filled syringe is for single patient only. Discard syringe after use. Do not reuse.
The content of un-opened and un-damaged blister is sterile, and must not be opened until use.
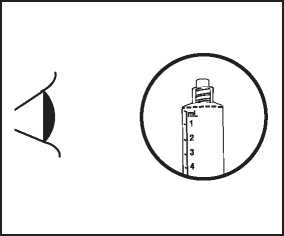
The product should be inspected visually for particles and discoloration prior to administration. Only clear colourless solution free from particles or precipitates should be used.
The product should not be used if the tamper evident seal on the syringe is broken.
The external surface of syringe is sterile until blister is opened.
When handled using an aseptic method, Phenylephrine 50 micrograms/ml, solution for injection in pre-filled syringe can be placed on a sterile field.
1) Withdraw the pre-filled syringe from the sterile blister.
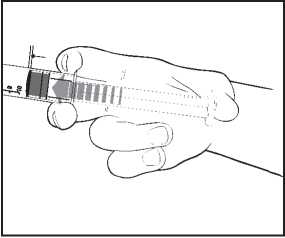
4) Check the syringe seal tip has been completely removed. If not, replace the cap and twist again.
2) Push on the plunger to free the bung. The sterilisation process may have caused adhesion of the bung to the body of the syringe.
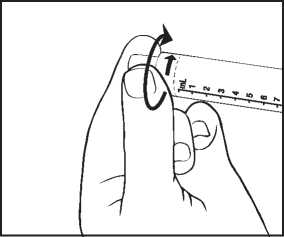
3) Twist off the end cap to break the seal. Do not touch the exposed luer connection in order to avoid contamination.
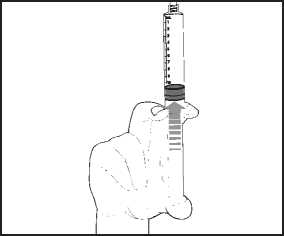
5) Expel the air by gently pushing the plunger.
6) Connect the syringe to the IV access. Push the plunger slowly to inject the required volume.
7) Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Package leaflet: Information for the patient
Phenylephrine 50 micrograms/ml, Solution for Injection in Pre-filled Syringe
(Referred to as “Phenylephrine Injection” in this leaflet) Phenylephrine
Read all of this leaflet carefully before you are given this
medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Phenylephrine Injection is and what it is used for
2. What you need to know before you are given Phenylephrine Injection
3. How Phenylephrine Injection is given
4. Possible side effects
5. How to store Phenylephrine Injection
6. Contents of the pack and other information
1. What Phenylephrine Injection is and what it is used for
This medicine belongs to a group of adrenergic and dopaminergic agents.
It is used to treat low blood pressure during anaesthesia.
2. What you need to know before you are given Phenylephrine Injection
You will not receive Phenylephrine Injection:
- if you are allergic to phenylephrine hydrochloride or any of the other ingredients of this medicine (listed in section 6);
- if you suffer from severe high blood pressure, or peripheral vascular disease (poor blood circulation);
- if you are taking a Monoamine Oxydase inhibitor (MAO) (or within 2 weeks of their withdrawal), that is used to treat depression (such as iproniazide, nialamide);
- if you suffer from severe overactive thyroid gland (hyperthyroidism).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Phenylephrine Injection:
- if you are elderly;
- if you are diabetic;
- if you suffer from arterial hypertension;
- if you have an overactive thyroid gland (uncontrolled hyperthyroidism);
- if you have blood vessel disease, such as arteriosclerosis (hardening and thickening of the walls of the blood vessels);
- if you have a poor blood circulation in the brain;
- if you suffer from heart disease including chronic heart conditions, peripheral vascular insufficiency, heart rhythm disorders, tachycardia (high heart rate), bradycardia (low heart rate), partial heart block, angina pectoris;
- if you suffer from a closed angle glaucoma (a rare eye disease).
In patients with serious heart failure, phenylephrine may worsen the heart failure as a consequence of blood vessel constriction.
The blood pressure in your arteries will be monitored during treatment. If you have heart disease, additional monitoring of vital functions will be performed.
Children
This medicine is not recommended for use in children due to insufficient data on efficacy, safety and dosage recommendations.
Other medicines and Phenylephrine Injection
Tell your doctor if you are using, have recently used or might use any other medicines, such as:
- certain antidepressants (iproniazid, nialamide, moclobenide, toloxatone, imipramine, milnacipran or venlafaxine);
- medicine used to treat infections (linezolid);
- certain medicines used to treat migraine (dihydroergotamine, ergotamine, methylergometrine, methylsergide);
- certain medicines used to treat Parkinson disease (bromocriptine, lisuride, pergolide);
- medicine used to inhibit the production of an hormone responsible of lactation (cabergoline);
- anaesthetics that are inhaled (desflurane, enflurane, halothane, isoflurane, methoxyflurane, sevoflurane);
- medicine used as an appetite suppressant (sibutramine);
- medicine used to treat high blood pressure (guanethidine);
- medicines used to treat heart failure and certain irregular heartbeats (cardiac glycosides);
- medicine used to treat abnormal heart rhythm (quinidine);
- medicine used during labour (oxytocin).
Pregnancy and breast-feeding
The safety of this medicine during pregnancy and breast-feeding has not been established, but the use of Phenylephrine Injection is possible during pregnancy if necessary.
Use of this medicine during breast-feeding is not recommended. However, in the event of a single administration during childbirth, breast-feeding is possible.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor, pharmacist or nurse for advice before being given this medicine.
Driving and using machines
Talk to your doctor if you want to drive and/or use machines after being given this medicine.
Phenylephrine Injection contains sodium
This medicinal product contains 3.68 mg (0.160 mmol) of sodium per ml (a total of 36.8 mg or 1.60 mmol sodium in a 10 ml syringe). This amount must be taken into consideration by patients on a salt-restricted diet.
3. How Phenylephrine Injection is given
The administration will be performed by a healthcare professional with appropriate training and relevant experience, who will decide the correct dosage for you and when and how the injection should be administered.
The recommended doses are:
Use in Adults
Your doctor will determine the dose to be administered, and may repeat or adjust it until the desired effect is attained.
Use in patients with impaired renal function (kidneys not functioning well)
Lower doses of phenylephrine may be needed in patients with impaired renal function.
Use in patients with impaired liver function (liver not functioning well) Higher doses of phenylephrine may be needed in patients with cirrhosis of the liver.
Use in older people
Treatment in older people should be carried out with care.
Use in children
This medicine is not recommended for use in children due to insufficient data on efficacy, safety and dosage recommendations.
If you have been given more Phenylephrine Injection than you should:
You may have the following symptoms: palpitation, cardiac rhythm disorders (tachycardia, cardiac arrhythmias).
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects may be serious (frequencies are not known). Tell your doctor straight away if you get any of the following:
- chest pain or pain due to the angina,
- irregular heartbeat,
- feeling the heart pumping in the chest,
- bleeding in the brain (speech disorder, dizziness, paralysis of one side of the body),
- psychosis (loosing contact with reality).
XXXXXX XX/XX
Other side effects may include (frequencies are not known):
- reaction of hypersensitivity (allergy),
- excessive dilation of the pupils,
- increased pressure in the eye (aggravation of glaucoma),
- excitability (excessive sensitivity of an organ or body part),
- agitation (restlessness),
- anxiety,
- confusion,
- headache,
- nervousness,
- insomnia (difficulty falling or staying asleep),
- shaking (tremor),
- burning of the skin,
- prickling of the skin,
- itching or tingling skin sensation (paresthesia),
- slow or high heart rate,
- high blood pressure,
- difficulty in breathing,
- fluids in the lung,
- nausea,
- vomiting,
- sweating,
- pallor or skin blanching (pale colour of the skin),
- goose flesh,
- tissue damage at the site of the injection,
- muscle weakness,
- difficulty in passing urine or urine retention.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via Yellow Card Scheme - Website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Phenylephrine Injection
Keep this medicine out of the sight and reach of children.
You should not be given this medicine after the expiry date which is stated on the carton and syringe label. The expiry date refers to the last day of that month. Your doctor or nurse will check this.
Store the blister in the outer carton in order to protect from light. Keep the syringe in its unopened blister until use.
Do not use this medicine if you notice visible signs of deterioration. Any syringe, even partially used, should be discarded appropriately after use.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Phenylephrine Injection contains
The active ingredient is phenylephrine hydrochloride.
- Each ml of solution for injection contains phenylephrine hydrochloride, equivalent to 50 micrograms phenylephrine.
- Each 10 ml pre-filled syringe contains phenylephrine hydrochloride, equivalent to 500 micrograms phenylephrine.
- The other ingredients are sodium chloride, sodium citrate dihydrate, citric acid monohydrate, sodium hydroxide, and water for injections.
What Phenylephrine Injection looks like and contents of the pack
Phenylephrine Injection is a clear colourless solution, in a 10 ml polypropylene pre-filled syringe, individually packaged in a transparent blister pack.
The pre-filled syringes are available in boxes of 1 and 10 syringes. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
LABORATOIRE AGUETTANT 1, rue Alexander Fleming 69007 LYON France
For any information about this medicine, please contact the local representative of the Marketing Authorisation Holder:
Aguettant Ltd
N°1, Farleigh House - Flax Bourton Bristol - BS48 1UR United Kingdom
This leaflet was last revised in 11/2015.
Detailed information on this medicinal product is available on the website of the Medicines and Healthcare Products Regulatory Agency (MHRA).
TECHNICAL LEAFLET
PHENYLEPHRINE 50 micrograms/ml, Solution for injection in pre-filled syringe
The following information is intended for medical and healthcare professionals only:
This is an extract from the Summary of Product Characteristics to assist in the administration of PHENYLEPHRINE 50 micrograms/ml, solution for injection in pre-filled syringe. When determining appropriateness of use in a particular patient, the prescriber should be familiar with the Summary of Product Characteristics of the product.
Qualitative and quantitative composition:
Each ml of solution for injection contains phenylephrine hydrochloride equivalent to 50 micrograms (0.05 mg) phenylephrine.
Each 10 ml pre-filled syringe contains phenylephrine hydrochloride equivalent to 500 micrograms (0.5 mg) phenylephrine.
Excipients with known effect: sodium.
Each ml of solution for injection contains 3.68 mg equivalent to 0.160 mmol of sodium.
Each 10 ml pre-filled syringe contains 36.8 mg equivalent to 1.60 mmol of sodium.
Other excipients: Sodium citrate dihydrate, Citric acid monohydrate, Sodium hydroxide, water for injections.
Description of solution:
Clear colorless solution. pH: 4.7 - 5.3
Osmolality: 270-300 mOsm/Kg
Posology and method of administration
Posology
Adults
Intravenous bolus injection:
Normal dose is 50 to 100 micrograms, which can be repeated until the desired effect is attained. One bolus dose should not exceed 100 micrograms.
Continuous infusion:
Initial dose is 25 to 50 micrograms/min. The doses may be increased or decreased to maintain the systolic blood pressure close to the normal value. Doses between 25 and 100 micrograms/min have been assessed to be effective.
Renal impairment
Lower doses of phenylephrine may be needed in patients with impaired renal function.
Hepatic Impairment
Higher doses of phenylephrine may be needed in patients with cirrhosis of the liver.
Older people
Treatment of the elderly should be carried out with care.
Paediatric population
The safety and efficacy of phenylephrine in children have not been established. No data are available.
Method of administration:
Parenteral administration. Intravenous bolus injection or intravenous infusion.
Phenylephrine, 50 micrograms/ml, solution for injection should only be administered by healthcare professionals with appropriate training and relevant experience.
Contraindications
Phenylephrine should not be used:
- hypersensitivity to the active substance or to any of the excipients listed in section 6.1;
- in patients with severe hypertension or peripheral vascular disease due to the risk of ischemic gangrene or vascular thrombosis;
- in combination with non-selective monoamine oxidase inhibitors (MAOs) (or within 2 weeks of their withdrawal) due to risk of paroxysmal hypertension and possibly fatal hyperthermia (see section 4.5);
- in patients with severe hyperthyroidism.
Special warning and precautions for use
The arterial blood pressure should be monitored during treatment. Phenylephrine should be administered with care to patients with:
- diabetes mellitus,
- arterial hypertension,
- uncontrolled hyperthyroidism,
- coronary heart disease and chronic heart conditions,
- non-severe peripheral vascular insufficiency,
- bradycardia,
- partial heart block,
- tachycardia,
- arrhythmias,
- angina pectoris (phenylephrine can precipitate or exacerbate angina in patients with coronary artery disease and history of angina),
- aneurysma,
- closed angle glaucoma.
Phenylephrine can induce a reduction in cardiac output. Therefore, care should be exercised in administering to patients with arteriosclerosis, the elderly and to patients with impaired cerebral or coronary circulation.
In patients with reduced cardiac output or coronary vascular disease, vital organ functions should be closely monitored and dose reduction should be considered when systemic blood pressure is near the lower end of the target range.
In patients with serious heart failure or cardiogenic shock, phenylephrine may cause deterioration in the heart failure as a consequence of the induced vasoconstriction (increase in afterload). Particular attention should be paid to Phenylephrine injection to avoid extravasation, since this may cause tissue necrosis.
This medicinal product contains sodium. Each 10 ml pre-filled syringe contains 36.8 mg (equivalent to 1.60 mmol of sodium). To be taken into consideration by patients on a controlled sodium diet.
Overdose:
Symptoms of overdose include headache, nausea, vomiting, paranoid psychosis, hallucinations, hypertension and reflex bradycardia. Cardiac arrhythmia such as ventricular extrasystoles and short paroxysmal episodes of ventricular tachycardia may occur. Treatment should consist of symptomatic and supportive measures. The hypertensive effects may be treated with an alpha-adrenoceptor blocking drug, such as phentolamine.
Pharmaceutical particulars:
Incompatibilities:
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
Storage:
Keep the syringe in its unopened blister until use. Store the blister in the outer carton in order to protect from light.