Phenylephrine 50 Micrograms/Ml Solution For Injection In Pre-Filled Syringe
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
PHENYLEPHRINE 50 micrograms/ml, solution for injection in pre-filled syringe
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution for injection contains phenylephrine hydrochloride equivalent to 50 micrograms (0.05 mg) phenylephrine.
Each 10 ml pre-filled syringe contains phenylephrine hydrochloride equivalent to 500 micrograms (0.5 mg) phenylephrine.
Excipients with known effect:
This medicinal product contains sodium.
Each ml of solution for injection contains 3.68 mg equivalent to 0.160 mmol of sodium.
Each 10 ml pre-filled syringe contains 36.8 mg equivalent to 1.60 mmol of sodium. For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Solution for injection in pre-filled syringe. (Injection)
Clear colourless solution. pH: 4.7 - 5.3
Osmolality: 270-300 mOsm/Kg
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Treatment of hypotension during spinal, epidural or general anesthesia.
4.2 Posology and method of administration
Posology
Adults
Intravenous bolus injection:
Normal dose is 50 to 100 micrograms, which can be repeated until the desired effect is attained. One bolus dose should not exceed 100 micrograms.
Continuous infusion:
Initial dose is 25 to 50 micrograms/min. The doses may be increased or decreased to maintain the systolic blood pressure close to the normal value. Doses between 25 and 100 micrograms/min have been assessed to be effective.
Renal impairment
Lower doses of phenylephrine may be needed in patients with impaired renal function.
Hepatic Impairment
Higher doses of phenylephrine may be needed in patients with cirrhosis of the liver.
Older people:
Treatment of the elderly should be carried out with care.
Paediatric population
The safety and efficacy of phenylephrine in children have not been established. No data are available.
Method of administration:
Parenteral administration. Intravenous bolus injection or intravenous infusion.
Phenylephrine, 50 micrograms/ml, solution for injection should only be administered by healthcare professionals with appropriate training and relevant experience.
4.3 Contraindications
Phenylephrine should not be used:
- hypersensitivity to the active substance or to any of the excipients listed in section 6.1;,
- in patients with severe hypertension or peripheral vascular disease due to the risk of ischemic gangrene or vascular thrombosis;
- in combination with non-selective monoamine oxidase inhibitors (MAOs) (or within 2 weeks of their withdrawal) due to risk of paroxysmal hypertension and possibly fatal hyperthermia (see section 4.5);
- in patients with severe hyperthyroidism.
4.4 Special warnings and precautions for use
The arterial blood pressure should be monitored during treatment.
Phenylephrine should be administered with care to patients with:
- diabetes mellitus;
- arterial hypertension;
- uncontrolled hyperthyroidism;
- coronary heart disease and chronic heart conditions;
- non-severe peripheral vascular insufficiency,
- bradycardia;
- partial heart block;
- tachycardia;
- arrhythmias;
- angina pectoris(phenylephrine can precipitate or exacerbate angina in patients with coronary artery disease and history of angina);
- aneurysma;
- closed angle glaucoma.
Phenylephrine can induce a reduction in cardiac output. Therefore, care should be exercised in administering to patients with arteriosclerosis, the elderly and to patients with impaired cerebral or coronary circulation.
In patients with reduced cardiac output or coronary vascular disease, vital organ functions should be closely monitored and dose reduction should be considered when systemic blood pressure is near the lower end of the target range.
In patients with serious heart failure or cardiogenic shock, phenylephrine may cause deterioration in the heart failure as a consequence of the induced vasoconstriction (increase in afterload).
Particular attention should be paid to Phenylephrine injection to avoid extravasation, since this may cause tissue necrosis.
This medicinal product contains sodium. Each 10 ml pre-filled syringe contains 36.8 mg (equivalent to 1.60 mmol of sodium). To be taken into consideration by patients on a controlled sodium diet.
4.5 Interaction with other medicinal products and other forms of interaction
Contraindicated combinations (see section 4.3)
• Non-selective monoamine oxidase inhibitors (MAOs) (iproniazid, nialamide)
Paroxysmal hypertension, hyperthermia possibly fatal. Due to the long duration of action of MAOIs, this interaction is still possible 15 days after discontinuation of the MAOI.
Inadvisable combinations
• Dopaminergic ergot alkaloids (bromocriptine, cabcrgolinc. lisuride. pcrgolidc): Risk of vasoconstriction and/or hypertensive crisis.
• Vasoconstrictor ergot alkaloids (dihydroergotamine. ergotamine. methylergometrine. methylsergide):
Risk of vasoconstriction and/or hypertensive crisis.
• Tricyclic antidepressants (e.g. imipramine):
Paroxysmal hypertension with possibility of arrhythmias (inhibition of adrenaline or noradrenaline entry in sympathetic fibers).
• Noradrenergic-serotoninergic antidepressants (minalcipram. venlafaxine):
Paroxysmal hypertension with possibility of arrhythmias (inhibition of adrenaline or noradrenaline entry in sympathetic fibers).
• Selective type A monoamine oxidase inhibitors (MAOs) (moclobemide. toloxatone)
Risk of vasoconstriction and/or hypertensive crisis.
• Linezolid:
Risk of vasoconstriction and/or hypertensive crisis.
• Guanethidine and related products:
Substantial increase in blood pressure (hyperreactivity linked to the reduction in sympathetic tone and /or to the inhibition of adrenaline or noradrenaline entry in sympathetic fibers). If the combination cannot be avoided. use with caution lower doses of sympathomimetic agents.
• Cardiac glycosides. quinidine:
Increased risk of arrhythmias.
• Sibutramine:
Paroxysmal hypertension with possibility of arrhythmias (inhibition of adrenaline or noradrenaline entry in sympathetic fibers).
• Halogenated volatile anaesthetics (desflurane. enflurane. halothane. isoflurane. methoxyflurane. sevoflurane):
Risk of perioperative hypertensive crisis and arrhythmia.
Combinations requiring precautions for use: • Oxytocic agents:
The effect of presso-active sympathomimetic amines is potentiated. Thus, some oxytocic agents may cause severe persistent hypertension and strokes can occur during post-partum period.
4.6 Fertility, pregnancy and lactation
Pregnancy
Animal studies are insufficient with respect to reproductive toxicity and teratogenicity (see section 5.3).
Administration of phenylephrine in late pregnancy or labour may potentially cause fetal hypoxia and bradycardia. Use of injectable phenylephrine is possible during pregnancy in accordance with the indications.
The combination with some oxytocic agents can cause severe hypertension (see section 4.5).
Breast-feeding
Small quantities of phenylephrine are excreted in human breast milk and oral bioavailability may be low.
Administering vasoconstrictors to the mother exposes the infant to a theoretical risk of cardiovascular and neurological effects. However, in the event of a single bolus administration during childbirth, breast-feeding is possible.
Fertility
There is no available data concerning fertility after exposure to phenylephrine (see section 5.3).
4.7 Effects on ability to drive and use machines
Not relevant.
4.8 Undesirable effects
Summary of the safety _ profile
The most common adverse events of phenylephrine are bradycardia, hypertensive episodes, nausea and vomiting. Hypertension is more frequent with high doses.
The most commonly reported cardiovascular adverse event appears to be bradycardia, likely due to baroreceptor-mediated vagal stimulation and consistent with the pharmacological effect of phenylephrine.
List of adverse reactions
Frequency: Not known (cannot be estimated from available data)
Immune system disorders:
Not known: hypersensitivity Psychiatric disorders:
Not known: Anxiety, excitability, agitation, psychotic states, confusion.
Nervous system disorders
Not known: Headache, nervousness, insomnia, paresthesia, tremor.
Eye disorders:
Not known: Mydriasis, aggravation of pre-existing angle-closure glaucoma Cardiac disorders:
Not known: Reflex bradycardia, tachycardia, palpitations, hypertension, arrhythmia, angina pectoris, myocardial ischemia.
Vascular disorders:
Not known: Cerebral haemorrhage, hypertensive crisis
Respiratory, thoracic and mediastinal disorders:
Not known: Dyspnoea, pulmonary oedema
Gastrointestinal disorders:
Not known: Nausea, vomiting
Skin and subcutaneous tissue disorders:
Not known: Sweating, pallor or skin blanching, piloerection, skin necrosis with extravasation
Musculoskeletal and connective tissue disorders:
Not known: muscular weakness
Renal and urinary disorders:
Not known: Difficulty in micturition and urinary retention Description of selected adverse reactions
As phenylephrine has been frequently used in the critical care setting in patients with hypotension and shock, some of the reported serious adverse events and deaths are probably related to the underlying disease and not related to the use of phenylephrine.
Other special population(s)
Elderly: risk for phenylephrine toxicity is increased in elderly patients (see section 4.4).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via Yellow Card Scheme
Website: www.mhra.gov .uk/vellowcard
4.9 Overdose
Svmptoms of overdose include headache, nausea, vomiting, paranoid psvchosis, hallucinations, hypertension and reflex bradycardia. Cardiac arrhythmia such as ventricular extrasystoles and short paroxysmal episodes of ventricular tachycardia may occur.
Treatment should consist of symptomatic and supportive measures. The hypertensive effects may be treated with an alpha-adrenoceptor blocking drug, such as phentolamine.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Adrenergic and dopaminergic agents, ATC Code: C01CA06
Phenylephrine is a potent vasoconstrictor that acts almost exclusively by stimulating alpha-1-adrenergic receptors. Such arterial vasoconstriction is also accompanied by venous vasoconstriction. This gives an increase in blood pressure and reflex bradycardia. The potent arterial vasoconstriction gives an increase in the systemic vascular resistance (increase in afterload). The overall result is a reduction in the cardiac output. This is less pronounced in healthy people but it may worsen in cases of previous heart failure. As Phenylephrine effects are linked to its pharmacological properties, they can be controlled by known antidotes.
5.2 Pharmacokinetic properties
The volume of distribution after single dose is 340 litres.
Phenylephrine is metabolised in the liver by monoamine oxidase.
Phenylephrine is mainly excreted via the kidneys as m-hydroxymandelic acid and phenol conjugates.
The duration of effect is 20 minutes after intravenous administration.
The terminal half life of injectable phenyleprine is about 3 hours.
The plasma protein binding is unknown.
There is no data available on the pharmacokinetics in special patient groups.
5.3 Preclinical safety data
There is no evidence of genotoxicity or carcinogenicity of phenylephrine. Animal studies are insufficient to evaluate effects on fertility and reproduction.
6.1 List of excipients
Sodium chloride,
Sodium citrate dihydrate,
Citric acid monohydrate,
Sodium hydroxide,
Water for injections
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
3 years
6.4 Special precautions for storage
Keep the syringe in its unopened blister until use. Store the blister in the outer carton in order to protect from light.
6.5 Nature and contents of container
10 ml polypropylene pre-filled syringe. The pre-filled syringes are available in box of 1 and 10 syringes.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Instructions for use:
Please prepare the syringe carefully as follows
The pre-filled syringe is for single patient only. Discard syringe after use. DO NOT REUSE.
The content of un-opened and un-damaged blister is sterile, and must not be opened until use.
The product should be inspected visually for particles and discoloration prior to administration. Only clear colourless solution free from particles or precipitates should be used.
The product should not be used if the tamper evident seal on the syringe is broken.
The external surface of syringe is sterile until blister is opened.
When handled using an aseptic method, Phenylephrine 50 micrograms/ml, solution for injection in pre-filled syringe can be placed on a sterile field.
1) Withdraw the pre-filled syringe from the sterile blister.
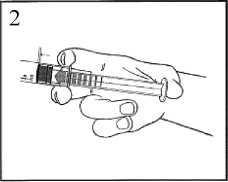
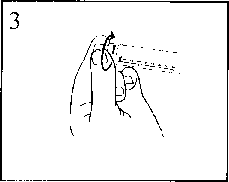
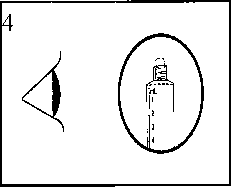
2) Push on the plunger to free the bung. The sterilisation process may have caused adhesion of the bung to the body of the syringe.
3) Twist off the end cap to break the seal. Do not touch the exposed luer connection in order to avoid contamination.
4) Check the syringe seal tip has been completely removed. If not, replace the cap and twist again
5) Expel the air by gently pushing the plunger.

6) Connect the syringe to the IV access. Push the plunger slowly to inject the required volume.
7 MARKETING AUTHORISATION HOLDER
Jenson Pharmaceutical Services Ltd
Carradine House
237 Regents Park Road
London
N3 3LF
United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 17871/0219
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
27/10/2015
10 DATE OF REVISION OF THE TEXT
27/10/2015