Polibar 94 % W/W Powder For Rectal Suspension
FPO
1. What Polibar is and what it is used for
PACKAGE LEAFLET: INFORMATION FOR THE USER
Polibar®
94 % w/w powder for rectal suspension
barium sulfate
Read all of this leaflet carefully before you have this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, radiographer or nurse helping you with your X-ray examination.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, radiographer or nurse helping you with your X-ray examination.
In this leaflet:
1. What Polibar is and what it is used for
2. Before you have Polibar
3. How Polibar will be given to you
4. Possible side effects
5. How to store Polibar
6. Further information
Talk to your doctor before you have Polibar if you:
• or members of your family suffer from allergies, hay fever, eczema or asthma
• are elderly
• are feeling weak (debilitated)
• are a baby or small child
• suffer from narrowing of the bowel (stenosis)
• suffer from a condition known as 'gastrointestinal fistulae'
• suffer from cancer of any part of the bowel
• suffer from inflammatory intestinal disease
• suffer from a condition known as 'diverticulitis' or 'diverticulosis'
• suffer from an infection known as 'amoebiasis'
• suffer from a condition called Hirschsprung's Disease
• have kidney problems
• suffer from constipation
• are dehydrated
• suffer from heart disease
• have been fitted with an artificial heart valve.
If any of the above applies to you, talk to your doctor before you have Polibar.
Using other medicines
Please tell your doctor, radiographer or nurse helping you with your X-ray examination if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Polibar may interfere with the action of other medicines taken at the same time. Your doctor may separate the administration of barium sulfate from that of your other medicines or other digestive tract examinations.
Polibar belongs to a group of medicines called 'contrast media'.
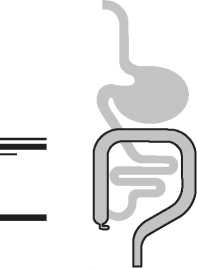
It is used when you have an X-ray of your digestive system. It contains a chemical that helps the X-ray show up. This gives a clearer 'picture' of your digestive system on the X-ray.
Polibar will be given to you as an enema (into your back passage).
This medicine is for diagnostic use only. It only helps to diagnose a problem. It cannot be used to treat any diseases.
Pregnancy and breast-feeding
Tell the doctor or nurse if you know that you are pregnant or you are trying to become pregnant. X-rays can harm unborn babies so you will only be given an X-ray if it is essential for you.
You can continue to breast feed if you take Polibar.
Driving and using machines
Polibar may cause you to feel dizzy. If this happens to you, do not drive or use machinery until this effect has worn off.
2. Before you have Polibar
3. How Polibar will be given to you
Do not have Polibar if you:
• are allergic (hypersensitive) to barium sulfate or any of the other ingredients of Polibar (see section 6)
• know that you have a hole or blockage in your bowel (gastrointestinal perforation or obstruction)
• know that you have an abnormal passage connecting your stomach with your bowel (gastrointestinal fistula)
• suffer from bleeding in your bowel
• suffer from insufficient blood supply (ischaemia) of the bowel wall
• have an enlarged bowel - a condition called megacolon or toxic megacolon
• have an inflamed condition of the bowel called necrotising enterocolitis
• suffer from poor movement of your bowel - a condition called colonic ileus
• have recently had bowel surgery, such as a procedure called 'hot' colonic biopsy or snare polypectomy
• have had radiotherapy (cancer treatment) to your large bowel (rectum) or prostate gland within the last 4 weeks
• have suffered recent injury to your bowel, including chemical burns.
If any of the above applies to you, you should not be given
Polibar. You should talk to your doctor again.
Adults, the elderly and children: Your doctor determines how much Polibar is used.
Polibar will be given to you as an enema (into your back passage). You will need to get into the best position for this.
You may be asked to lie on your side with one knee bent to your chest. A tube will be gently inserted into your back passage (anus). The medicine will then flow into your bowel.
If you are dehydrated, prone to suffering from constipation or elderly you may be offered a laxative before you use Polibar.
After your examination, you may be given a drink, laxative or put on a saline drip.
TX1628-1
Rev. 10/12 TX1628-1
The following information is intended for medical or healthcare professionals only.
It supplements the information provided in the package leaflet above.
Posology and method of administration
Polibar is for rectal (enema) administration. The powder must be reconstituted prior to administration (see section 'Special precautions for disposal and other handling' below).
The administered dose of Polibar will depend on the patient in question and the section of the gastrointestinal tract to be viewed.
Adults: Usual Dosage Range 150 - 750 g barium sulfate in a suitable suspension.
Double contrast of the large bowel - Give as required between 60 -115 % w/v.
Single contrast of the large bowel - Give as required between 20- 40 % w/v.
The actual administered dose should be determined, from experience, by the radiologist.
Children: The dosage will be dependent on the size, age, health state and anatomic region to be imaged of the child. Individual requirements should be determined, from experience, by the radiologist.
Elderly: There are no special dosage recommendations. The dosage should be determined, from experience, by the radiologist.
Special precautions for disposal and other handling
Adults: Polibar should be suspended over the density range of 20 - 115 % w/v (20 - 60 % w/w).
Reconstitution information for use of Polibar is provided below.
Odd
6. Further information
4. Possible side effects
5. How to store Polibar
Like all medicines, Polibar can cause side effects, although not everybody gets them.
Seek immediate medical help if you have any of the following symptoms:
• feeling faint or loss of consciousness
• swelling of the face or throat
• difficulty in breathing or wheezing, shortness of breath.
These are the signs of a severe and sometimes life threatening allergic reaction, such as shock.
The following side effects can also develop when you are given this type of medicine:
Skin problems:
• itching
• redness
• rash
• sweating
• pale, clammy skin
• skin turning a blue or purple colour due to poor circulation.
Stomach/bowel problems:
• constipation
• blocked bowel (the blockage would have to be removed by a doctor)
• diarrhoea
• feeling sick or being sick
• stomach pain or a bloated stomach
• excess wind (flatulence)
• bowel inflammation, ulceration or perforation (a hole)
• swollen tongue
• a damaged bowel wall lining which may lead to bacteria in the blood, an abscess or appendicitis. You may be given antibiotics to prevent this.
• in rare cases an enema may damage the lining of the bowel wall. When this happens, it may result in an infection of the bowel or its lining (peritonitis), or a type of swelling called 'granuloma'
• small amounts of barium sulfate may leak into the blood supply and end up in other parts of the body such as blood vessels or arteries. This occurs rarely, but the result can be very serious and may cause death.
• if you suffer from ulcerative colitis (inflammation of the bowel), Polibar may aggravate your condition.
Heart problems:
• changes in heart rhythm.
Respiratory problems:
• difficulty breathing
• cough and sore throat.
Other possible side effects:
• high blood sugar (hyperglycaemia) in diabetics
• agitation, confusion or nervousness whilst the product is being administered
• feeling dizzy
• swollen eyes
• tinnitus (ringing in the ears)
• low blood pressure
• problems passing urine
• feeling unwell, pain including headache, fever
• swelling, weakness, muscle or speech problems.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor.
Polibar should be kept out of the reach and sight of children.
Do not use Polibar after the expiry date which is stated on the packaging behind the word 'EXP'. The expiry date refers to the last day of that month.
Your doctor or nurse will make sure your medicine is correctly stored and disposed of.
What Polibar contains
• The active ingredient is barium sulfate 94 % w/w.
• The other ingredients are gum ghatti, sorbitol (E420), sodium citrate (E331), sodium carrageenan (E407), simeticone, polyoxyethylene glyceryl mono-oleate, and citric acid anhydrous (E330).
What Polibar looks like and contents of the pack
Polibar is a white powder for rectal suspension. It is supplied in enema bags containing 397 g, 567 g or 680 g Polibar. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer responsible for batch release in the EU
Bracco UK Limited,
Wooburn Green, Bucks, HP10 0HH, UK
If this leaflet is difficult to see or read, and you would like it in a different format, please contact Bracco UK Limited, Wooburn Green, Bucks, HP10 0HH, UK.
This leaflet was last approved in 10/2012.
680 g enema bag
|
Polibar Density Chart (Ghatti) | ||
|
0 40 60 80 100 120 | ||
Attach clamp to tubing and close. Study the appropriate graph below. This shows the density range and how much water to use for each density. Measure the indicated quantity of warm (40 oC) water and add this to the bag through the red snap-cap seal. Hold the bag by the finger holes and shake vigorously for 10-20 seconds. When ready to use shake again, 1 0-20 seconds. Then with the thumb and forefinger pop the red ball at the bag tube junction into the bag. Run barium through the tubing - attach rectal tube. The kit is now ready.
Water to be added (mL)
This pack is for single-dose use only. Polibar should be administered immediately following reconstitution and must not be stored.
397 g enema bag 567 g enema bag
Polibar Density Chart (Ghatti)
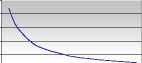
30 50 70 90 110 130
% Barium Sulfate (w/v)
Polibar Density Chart (Ghatti)
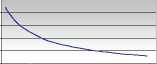
20 40 60 80 100 120
Special precautions for storage
Store below 25 °C. Store in the original package.
Any unused, opened product or waste material should be disposed of in accordance with local requirements.
LO
CD
<
00
h-
TX1629-1
94 % w/w barium sulfate 24 enema bags of 397 g
Each enema bag contains 397 g powder which contains 373 g barium sulfate
List of Excipients: gum ghatti, sorbitol (E420), sodium citrate (E331), sodium carrageenan (E407), simeticone, polyoxyethylene glyceryl mono-oleate, citric acid anhydrous (E330)
Important: Read the leaflet before use
Instructions for use: For rectal (enema) administration
KEEP OUT OF REACH AND SIGHT OF CHILDREN
Store below 25 °C. Store in the original package. Do not store reconstituted product.
Marketing Authorisation Holder: Bracco UK Limited, Wooburn Green, Bucks, HP10 OHH, UK
PL 18920/0014 rev. 03/12
0
94 % w/w barium sulfate 24 enema bags of 397 g
Each enema bag contains 397 g powder which contains 373 g barium sulfate
List of Excipients: gum ghatti, sorbitol (E420), sodium citrate (E331), sodium carrageenan (E407), simeticone, polyoxyethylene glyceryl mono-oleate, citric acid anhydrous (E330)
Important: Read the leaflet before use
Instructions for use: For rectal (enema) administration
KEEP OUT OF REACH AND SIGHT OF CHILDREN
Store below 25 °C. Store in the original package. Do not store reconstituted product.
Marketing Authorisation Holder:
Bracco UK Limited,
Wooburn Green, Bucks, HP10 OHH, UK PL 18920/0014
+H784AG1H5/
CM
O
<
CO
h-
TX1631-1
94 % w/w barium sulfate 24 enema bags of 567 g
Each enema bag contains 567 g powder which contains 533 g barium sulfate
List of Excipients: gum ghatti, sorbitol (E420), sodium citrate (E331), sodium carrageenan (E407), simeticone, polyoxyethylene glyceryl mono-oleate, citric acid anhydrous (E330)
Important: Read the leaflet before use
Instructions for use: For rectal (enema) administration
KEEP OUT OF REACH AND SIGHT OF CHILDREN
Store below 25 °C. Store in the original package. Do not store reconstituted product.
Marketing Authorisation Holder: Bracco UK Limited, Wooburn Green, Bucks, HP10 OHH, UK
PL 18920/0014 rev. 03/12
0
94 % w/w barium sulfate 24 enema bags of 567 g
Each enema bag contains 567 g powder which contains 533 g barium sulfate
List of Excipients: gum ghatti, sorbitol (E420), sodium citrate (E331), sodium carrageenan (E407), simeticone, polyoxyethylene glyceryl mono-oleate, citric acid anhydrous (E330)
Important: Read the leaflet before use
Instructions for use: For rectal (enema) administration
KEEP OUT OF REACH AND SIGHT OF CHILDREN
Store below 25 °C. Store in the original package. Do not store reconstituted product.
Marketing Authorisation Holder:
Bracco UK Limited,
Wooburn Green, Bucks, HP10 OHH, UK PL 18920/0014
+H784AC2H5.
D
TXxxxx
94 % w/w barium sulfate 24 enema bags of 680 g
Each enema bag contains 680 g powder which contains 639 g barium sulfate
List of Excipients: gum ghatti, sorbitol (E420), sodium citrate (E331), sodium carrageenan (E407), simeticone, polyoxyethylene glyceryl mono-oleate, citric acid anhydrous (E330)
Important: Read the leaflet before use
Instructions for use: For rectal (enema) administration
KEEP OUT OF REACH AND SIGHT OF CHILDREN
Store below 25 °C. Store in the original package. Do not store reconstituted product.
Marketing Authorisation Holder: Bracco UK Limited, Wooburn Green, Bucks, HP10 OHH, UK
PL 18920/0014 rev. 03/12

94 % w/w barium sulfate 24 enema bags of 680 g
Each enema bag contains 680 g powder which contains 639 g barium sulfate
List of Excipients: gum ghatti, sorbitol (E420), sodium citrate (E331), sodium carrageenan (E407), simeticone, polyoxyethylene glyceryl mono-oleate, citric acid anhydrous (E330)
Important: Read the leaflet before use
Instructions for use: For rectal (enema) administration
KEEP OUT OF REACH AND SIGHT OF CHILDREN
Store below 25 °C. Store in the original package. Do not store reconstituted product.
Marketing Authorisation Holder:
Bracco UK Limited,
Wooburn Green, Bucks, HP10 OHH, UK PL 18920/0014
TXxxxx rev. 03/12