Prevora 100 Mg/Ml Dental Solution

Package Leaflet: Information for the User
Prevora 100 mg/ml Dental Solution Chlorhexidine diacetate
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have further questions, please ask your dental professional.
• This coating has been recommended for your teeth by your dental professional and will be applied to your teeth by your dental professional on several occasions.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your dental professional.
In this leaflet
1. What Prevora is and what it is used for.
2. Before you are treated with Prevora.
3. How you are treated with Prevora.
4. Possible side effects.
5. How to store Prevora.
6. Further information.
1. WHAT PREVORA IS AND WHAT IT IS USED FOR
This dental coating is a topical treatment for the prevention of cavities on the crown and the root of the tooth of adult patients at high-risk of tooth decay (e.g. dry mouth sufferers or those with a number of cavities when you visit the dentist). This coating temporarily covers your teeth to reduce the bacteria on your teeth which cause tooth decay.
Oral hygiene and sugar intake: If you have poor oral hygiene and/or you frequently consume sugars you need to make sure you brush your teeth with fluoridated tooth paste and control your sugar intake, as they are important contributors to the overall success of Prevora.
2. BEFORE YOU ARE TREATED WITH PREVORA
Special warnings and precautions
Prevora may in rare cases cause severe allergic reactions, leading to a drop in blood pressure and even to unconsciousness. Early symptoms of a severe allergic reaction may be skin rash or asthma. If you notice these symptoms, stop using Prevora and contact your doctor as soon as possible (see under section 4: "Possible side effects")
Do not take/use Prevora
• If you are allergic (hypersensitive) to chlorhexi-dine gluconate^Sumatra benzoin or ethanol (see section 6).
• If you are allergic to the ingredients of the Stage 2 sealant, which is a secondary coating applied immediately over Stage 1. The ingredients of this second coating are methacrylate, triethyl citrate and purified water.
• If you have been treated with a fluoride dental varnish in the past 3 days.
Take special care with Prevora
• before undergoing treatment with Prevora, to report your medical condition including asthma, eczema and other allergies, to your dentist.
Taking other medicines
Please inform your dental professional if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breast-feeding
Ask your dentist for advice before taking or receiving any medicine.
Driving and using machines
Prevora has not been shown to affect driving.
Prevora has not been shown to affect the use of machines.
Important information about some of the ingredients of Prevora
This dental coating can cause a temporary irritation or stinging of the gums, lips or tongue and can also have a bitter taste. Chlorhexidine, the active substance in Prevora, can also cause staining of the teeth; however, when chlorhexidine is applied as a topical, temporary dental coating, staining is rare.
3. HOW YOU ARE TREATED WITH PREVORA
This topical, temporary coating on your teeth is applied by your dental professional in a short appointment in the dental office. The dental professional will first clean your teeth, and then apply Stage 1 to all tooth surfaces (Figure 1), followed immediately by a second coating of Stage 2. This second coating temporarily protects Stage 1 from your saliva and from food abrasion.
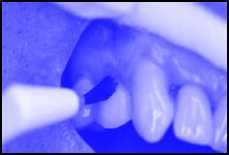
Figure 1 - Stage 1 followed by a second coating will be applied to your teeth so as to reduce your risk of tooth decay.
At the start of your treatment with Prevora, you will require 4 weekly treatments followed by another single treatment in 6 months. Thereafter, your need for more treatments with Prevora will be evaluated by your dental professional.
You will be told by your Dental Professional that:
• The dried Prevora coating will begin coming off the teeth during the next meal
• You should avoid eating hard foods (e.g. meat, apples) for at least 4 hours after treatment.
• You should not chew gum for at least 24 hours.
• You should not brush your teeth for 24 hours after treatment. Then you resume brushing with a new brush 2 to 3 times daily with fluoride tooth paste.
• You should not floss for 3 days after treatment. Then you resume flossing daily.
• If dentures are worn, clean and disinfect these dentures regularly prior to use. Disinfect using soap and warm water.
Effects when the Prevora treatment is finished
Temporarily, you may have a bitter taste, a sensation of a coating on your teeth and/or a stinging or burning along your gum line or tongue. The stinging will last for a few minutes, the coating for a few hours. The coating will be invisible on your teeth. Chlorhexidine, the active substance in Prevora , can stain the teeth when used in a mouth rinse or gel; however, in controlled clinical studies and dental office use, the occurrence of tooth staining by the Prevora dental coating is rare.
If staining occurs it is not permanent and can largely be eliminated by brushing with a conventional fluoridated toothpaste, once tooth brushing re-commences 24 hours after treatment with Prevora. A professional cleaning will remove any staining but if conducted within 7 days of treatment will also minimize the effect of Prevora. If you receive a professional cleaning, it should be done 7 days after the Prevora treatment.
What to do after you are treated with Prevora
To preserve this coating on your teeth for as long as possible, eat soft foods at your next meal (e.g. soup). Do not eat hard foods (e.g. meat, apples) for at least 4 hours after treatment. Do not chew gum for at least 24 hours.
Do not brush your teeth for 24 hours after this treatment. Then resume brushing with a new tooth brush and brush 2 to 3 times daily with fluoride toothpaste.
Do not floss your teeth for 3 days following this treatment. Then resume flossing daily.
If dentures are worn, clean and disinfect at home prior to use. Disinfect using soap and warm water.
Make sure you receive all the treatments of Prevora, recommended by your dentist.
It is an important contribution to the overall success of this treatment, that you regularly brush your teeth with a fluoridated tooth paste, and that you control your consumption of foods and drinks which have a high amount of sugar and acid.
If you have any further questions on the use of this product, ask your dental professional.
4. POSSIBLE SIDE EFFECTS
If you experience any of the following reactions stop using Prevora and get immediate help:
swelling of the face, lips, tongue or throat; a red itchy skin rash; wheezing or difficulty breathing; feeling faint and dizzy; a strange metallic taste in the mouth; collapse. You may be having an allergic reaction.
If you develop a rash or your skin becomes itchy, red, dry or inflamed where you have used the product as a skin wash, stop using it and talk to your doctor or pharmacist.
Like all medicines, Prevora can cause side effects, although not everybody gets them.
If you have prolonged stinging or burning of the gums, lips or tongue, you should see your dental professional as soon as possible. If your teeth appear stained after treatment with Prevora, please inform your dental professional.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your dental professional.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE PREVORA
Keep out of the reach and sight of children.
Do not use Prevora after the expiry date which is stated on the carton and single vial labels after EXP. The expiry date refers to the last day of that month.
Discard any remaining solution immediately after use.
Prevora should be stored in a refrigerator (2°C - 8°C) at your dental professional's office.
Medicines should not be disposed of via wastewater or household waste. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Prevora contains:
Stage 1 chlorhexidine coating:
100 mg/ml Dental Solution
The active substance is Chlorhexidine diacetate.
The other ingredients are Sumatra benzoin and Ethanol
Stage 2 sealant coating:
The ingredients are Ammonio methacrylate copolymer dispersion, Type B and Triethyl citrate
What Prevora looks like and contents of the pack:
Dental Solution
The chlorhexidine coating is a clear, slightly brownish solution with a noticeable scent, free of any floating particles in the solution.
The sealant coating is a milky-white liquid with a faint scent
Each treatment set of Prevora 100 mg/ml Dental Solution consists of:
6 vials of Stage 1 chlorhexidine coating in a 2 ml Type 1 glass vial
6 vials of Stage 2 sealant coating in a 2 ml Type 1 glass vial
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
CHX Technologies Europe Limited Guinness Enterprise Centre Taylor's Lane Dublin 8, Ireland Tel: (01) 4100600
Manufacturer:
Jenson Pharmaceutical Services Ltd.
Carradine House 237 Regents Park Road London N3 3LF United Kingdom
This leaflet was last approved in June 2014.