Prevora 100 Mg/Ml Dental Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Prevora 100mg/ml Dental Solution.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each 1 ml of Prevora Dental Solution (Stage 1 chlorhexidine coating) contains chlorhexidine diacetate 100mg
For a list of full excipients, see section 6.1
3 PHARMACEUTICAL FORM
Dental Solution
Stage 1 chlorhexidine coating
A clear, slightly brownish solution with a characteristic odour, free of visible particulate matter.
Stage 2 sealant coating
A milky-white liquid of low viscosity with a faint characteristic odour, free of visible particulate matter.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Prevora 100mg/ml Dental Solution is an antiseptic solution which is applied topically to the dentition of patients for the prevention of coronal and root caries in adult patients at high-risk of dental caries (e.g. xerostomia sufferers or those with 3 or more caries at the start of the treatment plan). To be used in dental offices only by dental professionals.
4.2 Posology and method of administration
Posology
An individual dosing in adults is between 300 ^l and 600 ^l of Prevora 100 mg/ml Dental Solution. The patient is to receive 5 treatments in the initial year of treatment, of which 4 are administered one week apart in the first month, and the final dose is administered at 6 months. Treatment of the dental patient thereafter is according to professional clinical judgment of the risk of dental caries.
Prevora 100 mg/ml Dental Solution is administered topically to the entire dentition of the patient using a cotton pellet or fine paint brush. The cotton pellet or brush is dipped into the Prevora 100 mg/ml Dental solution and thereafter is applied to the tooth surfaces (Figure 1).
Figure 1 Application of Prevora 100 mg/ml Dental Solution
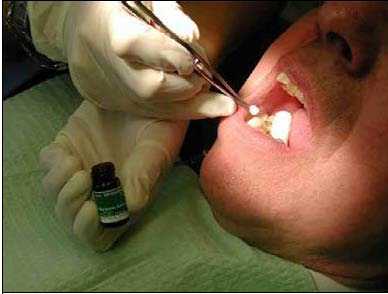
Dip cotton pellet or fine brush in vial and then apply pellet or brush to tooth surfaces.
The patient should be instructed that:
• The dried Prevora coating will begin coming off the teeth during the next meal.
• They should avoid eating hard foods (e.g. meat, apples) for at least 4 hours after treatment
• Do not chew gum for at least 24 hours.
• Do not brush his/her teeth for 24 hours after treatment. Then to resume brushing with a new brush 2 to 3 times daily with fluoride tooth paste.
• Do not floss for 3 days after treatment. Then to resume flossing daily.
• If dentures are worn, clean and disinfect these dentures regularly prior to use. Disinfect using soap and warm water.
Method of administration
External (oral) topical use in the dental office by a dental professional. This product is not intended to be swallowed.
4.3 Contraindications
Known hypersensitivity to chlorhexidine, Sumatra benzoin or ethanol, especially in those with a history of possible chlorhexidine-related allergic reactions (see sections 4.4 and 4.8)
4.4 Special warnings and precautions for use
Prevora 100 mg/ml Dental Solution contains chlorhexidine. Chlorhexidine is known to induce hypersensitivity, including generalised allergic reactions and anaphylactic shock. The prevalence of chlorhexidine hypersensitivity is not known, but available literature suggests this is likely to be rare. Prevora 100 mg/ml Dental Solution should not be administered to anyone with a potential history of an allergic reaction to chlorhexidine-containing compound (see sections 4.3 and 4.8)
For external (oral) topical use only - keep out of the eyes and ears. If the drug product comes into contact with the eyes, wash out promptly and thoroughly with water.
Prevora 100 mg/ml Dental Solution should be used with caution in patients with a history of asthma or eczema. Avoid application of Prevora 100 mg/ml Dental Solution to the soft tissues. Failure to do so can result in temporary stinging or mild inflammation of the soft tissues.
4.5 Interaction with other medicinal products and other forms of interaction
Prevora 100 mg/ml Dental Solution should not be applied immediately following use of an oil-based prophylactic paste or up to 3 days following application of a fluoride dental varnish.
Chlorhexidine is incompatible with anionic agents.
4.6 Pregnancy and lactation
No controlled studies have been carried out to ascertain if there are any adverse reactions when Prevora 100 mg/ml Dental Solution is applied to the dentition of women of childbearing potential, or to the dentition of expectant or breast feeding mothers. Therefore, it is recommended that Prevora 100 mg/ml Dental Solution should not be administered during pregnancy. Since many drugs are excreted during lactation and there have not been any studies performed using Prevora 100 mg/ml Dental Solution in nursing mothers, it is recommended that Prevora 100 mg/ml Dental Solution should not be applied if the mother is nursing.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
4.8 Undesirable effects
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
|
Uncommon (>1/1,000 to <1/100) |
Not known (cannot be estimated from the available data) | |
|
Skin and subcutaneous tissues disorders |
Redness and/or temporary stinging sensation of the oral mucosa Objectionable, bitter taste when Prevora 100 mg/ml Dental Solution comes into contact with the saliva or oral mucosa |
Frequency not known: Allergic skin reactions such as dermatitis, pruritus, erythema, eczema, rash, urticaria, skin irritation and blisters. Immediate hypersensitivity reactions to chlorhexidine (urticaria or anaphylaxis) |
|
General disorders and administration site conditions |
Immune Disorders: Frequency not known: Hypersensitivity including anaphylactic shock (see sections 4.3 and 4.4) Transient tooth sensitivity and loss of taste Discoloration of the teeth and silicate or composite restorations |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
4.9 Overdose
There is no experience with over-dosage with Prevora 100 mg/ml Dental Solution. Consequently, the signs and symptoms have not been identified. If overdose should occur, treat symptomatically.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: A01AB Anti-infectives and antiseptics for local oral treatment.
ATC Code: A01AB03 chlorhexidine.
Chlorhexidine is effective against a wide range of important oral microorganisms associated with dental caries. Chlorhexidine in the drug product has been found at bactericidal levels to Streptococcus mutans for between 24 hours and 48 hours on the surface of adult dental patients after its application, as measured by HPLC.
There have been no published reports of permanent resistance by Streptococcus mutans to the repeated use of chlorhexidine for up to 2 years and no significant
resistance to Streptococcus mutans or opportunistic infections with Candida albicans were observed after treatment with Prevora over one year in adult patients. The cumulative monthly mean dose of chlorhexidine delivered by Prevora 100 mg/ml Dental Solution is approximately equal to that of 1.0% w/w chlorhexidine dental gel and approximately half that of 0.2% w/v chlorhexidine oral rinse.
5.2 Pharmacokinetic properties
Chlorhexidine binds strongly to the oral mucosa and the dentition and thus has very poor systemic absorption. No detectable blood levels of chlorhexidine have been found after oral use.
5.3 Preclinical safety data
Preclinical data reveal no special hazard for humans.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Stage 1 chlorhexidine coating:
Sumatra benzoin Ethanol
Stage 2 sealant coating:
Ammonio methacrylate copolymer dispersion, Type B Triethyl citrate
6.2 Incompatibilities
In the absence of compatibility studies, this topical medicinal product must not be mixed with other topical medicinal products.
6.3 Shelf life
18 months.
Discard any remaining solution immediately after use.
6.4 Special precautions for storage
Store in refrigerator (2° to 8°C)
6.5 Nature and contents of container
Prevora 100 mg/ml Dental Solution contains:
Stage 1 chlorhexidine coating:
Chlorhexidine diacetate Sumatra benzoin Ethanol
Stage 2 sealant coating:
Ammonio methacrylate copolymer dispersion, Type B Triethyl citrate
One treatment box of Prevora Dental solution contains 6 Type 1 glass vials of Stage 1 chlorhexidine coating along with 6 Type 1 glass vials of Stage 2 sealant coating.
6.6 Special precautions for disposal
Step #1. Preparation: Ensure that the dentition contains no open caries lesions or restorations with imperfect margins. Prepare for the application with a tray (Figure 2), consisting of cotton rolls, cotton pellets or fine brushes, forceps, air syringe, and a vial of Stage 1 chlorhexidine coating and a vial of Stage 2 sealant coating.
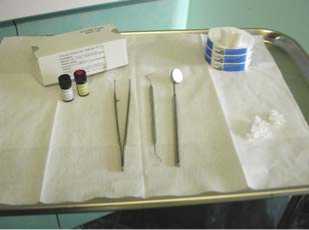
Figure 2: Tray set up for Stage
One Stage 1 chlorhexidine coating and Stage 2 sealant coating treatments.
Step #2. Prophylaxis: Give a rubber cup prophylaxis using flour of pumice and water. Avoid using a non-oil based prophylactic paste.
Step #3. Floss: Thoroughly rinse and floss the patient’s teeth with un-waxed floss to remove pumice and residual dental plaque. Ensure the cleanliness of the distal surface of the last tooth in each arch by wiping it with a cotton pellet held in a pair of forceps.
Step #4. Isolate one quadrant: Isolate one quadrant of the dentition with cotton rolls and a saliva ejector.
Step #5. Dry teeth: Dry all teeth in that quadrant with an air syringe.
Step #6. Apply Stage 1 chlorhexidine coating inter-proximallv: Using a cotton pellet held in forceps, or a fine brush suitable for reaching inter-proximal areas, apply Stage 1 Chlorhexidine coating to the inter-proximal areas of all posterior teeth in the quadrant, ensuring not to apply the coating to the soft tissues. Then dry these tooth surfaces with an air syringe.
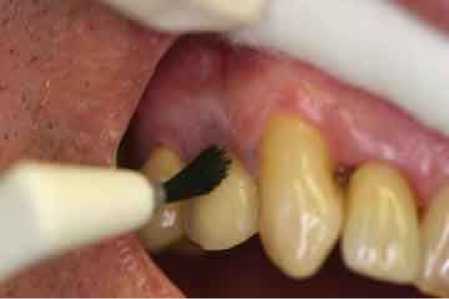
Figure 3: Apply Prevora Stage 1 chlorhexidine coating with a fine brush to the gingival margin, followed by Prevora Stage 2 sealant coating using the same techniques.
Step #7. Apply Stage 1 chlorhexidine coating to other tooth surfaces: Apply this coating to all other tooth surfaces (Figure 3) in this same quadrant, and then air dry. Be careful to avoid applying Stage 1 chlorhexidine coating to the soft tissues as the patient may experience stinging or burning of the gums or tongue.
Step #8. Apply Stage 2 sealant coating: Apply this second coating (with white cap), using a second cotton pellet or with another fine brush, to this same quadrant. Then dry this second coating with an air syringe.
Step #9. Repeat coating of other quadrants: Repeat steps 4 through 8 in the remaining quadrants of the dentition.
Step #10. Advise the patient:
Instruct the patient:
• The dried Prevora coating will begin coming off the teeth during the next meal.
• To avoid eating hard foods (e.g. meat, apples) for at least 4 hours after treatment.
• Do not chew gum for at least 24 hours.
• Do not brush his/her teeth for 24 hours after treatment. Then to resume brushing with a new brush 2 to 3 times daily with fluoride tooth paste.
• Do not floss for 3 days after treatment. Then to resume flossing daily.
• If dentures are worn, clean and disinfect these dentures regularly prior to use. Disinfect using soap and warm water.
Step #11. Schedule repeat treatments: Repeat this initial Prevora application every week for 3 more weeks after the initial application, followed by a single application at six months and thereafter according to clinical judgment.
Instruments and clothing in contact with Stage 1 chlorhexidine coating may be cleaned with alcohol. Instruments and clothing in contact with Stage 2 sealant coating may be cleaned with water.
Any unused product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
CHX Technologies Europe Limited Guinness Enterprise Centre Taylor’s Lane Dublin 8 Ireland
8 MARKETING AUTHORISATION NUMBER(S)
PL 30669/0001
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
04/05/2011
10 DATE OF REVISION OF THE TEXT
23/09/2014