Rabipur Powder And Solvent For Solution For Injection In Pre-Filled Syringe
|
Change request |
301480 |
|
Comm. name/ country |
Rabipur GB |
|
Pack. mat. |
leaflet |
|
Component code |
MCK14-137 |
|
Barcode (nr) |
N/A |
|
Dimensions |
158x560/280mm |
|
Colours |
Black |
|
Draft |
3 |
PACKAGE LEAFLET: INFORMATION FOR THE USER
RABIPUR®
Powder and solvent for solution for injection in pre-filled syringe
Rabies virus (Inactivated, strain Flury LEP)
Read all of this leaflet carefully before you/your child receives Rabipur. It contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed for you/your child only. Do not pass it on to others.
- If you get any side effects, talk to your doctor, pharmacist, or nurse.
This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Rabipur is and what it is used for
2. What you need to know before you/your child receives Rabipur
3. How to use Rabipur
4. Possible side effects
5. How to store Rabipur
6. Contents of the pack and other information
1. WHAT RABIPUR IS AND WHAT IT IS USED FOR What Rabipur is
Rabipur is a vaccine containing rabies virus that has been killed. After administration of the vaccine, the immune system (the body's natural defence system) forms antibodies to rabies viruses. These antibodies protect from infections or diseases by the virus that causes rabies. None of the components of the vaccine can cause rabies.
What Rabipur is used for
Rabipur can be used in individuals of all ages.
Rabipur can be used in 2 ways:
• to prevent rabies before possible risk of exposure to rabies virus (pre-exposure prophylaxis). or
• to treat people after suspected or proven exposure to rabies virus (post-exposure prophylaxis).
Rabies is an infection that can be transmitted when a person is bitten, scratched or even just licked by an infected animal, particularly when the skin is already injured. Even contact with animal traps that were licked or bitten by infected animals can cause infections in humans.
2. WHAT YOU NEED TO KNOW BEFORE YOU/YOUR CHILD RECEIVES RABIPUR
You/Your child must not receive Rabipur before possible risk of exposure to the rabies virus if you/your child:
• Have/has a history of having a severe allergic reaction to any ingredient of the vaccine listed in section 6.
• Have/has an acute disease requiring treatment. Vaccination is usually postponed until at least 2 weeks after recovery. The presence of a minor infection, such as a cold, should not require postponement of the vaccination, but talk to your doctor or nurse first
After suspected or proven exposure to rabies virus you/your child may be given Rabipur if you/ your child:
• Are/is allergic to any ingredient of the vaccine, or has an acute disease requiring treatment. This is because rabies is such a serious disease.
• Pregnant or breastfeeding women may be vaccinated with Rabipur to treat rabies infection after suspected or proven exposure to rabies virus.
Severe allergic reactions (hypersensitivity)
If you or your child is known to be at risk of a severe allergic reaction to the vaccine or to any of the ingredients, you/your child may be given a different vaccine against rabies that does not contain these ingredients. If there is no alternative vaccine available, your doctor or nurse will discuss the risks of vaccination and rabies virus infection with you before you or your child receives the vaccine.
Warnings and precautions
Severe allergic reactions including anaphylactic shock (a life-threatening allergic response involving the whole body and in which blood pressure falls dangerously low) have occurred following Rabipur vaccination. As with all injectable vaccines, appropriate medical treatment and supervision should always be readily available in case of a rare severe allergic reaction to the vaccine.
Talk to your doctor or nurse before you or your child receives Rabipur if you/your child:
• Have/has a severe allergy to egg or egg products (for symptoms see section 4 of this leaflet). Rabipur contains residues of chicken proteins left over from the manufacturing process.
• Have/has a severe allergy to the antibiotics neomycin, chlortetracycline, or amphotericin B. These antibiotics may be present in very small amounts in the vaccine.
• Have/has a severe allergy to polygeline.
Cases of very rare but severe conditions affecting the nervous system have been reported following the receipt of Rabipur vaccine. See section 4. Anti-inflammatory medicines (steroids), often used to treat these conditions, may interfere with the effectiveness of the vaccine (see below, other medicines and Rabipur). Your doctor or nurse will decide how to proceed in this circumstance.
Other medicines and Rabipur
Tell your doctor or nurse if you are /your child is taking, have/has recently taken, or plan to take any other medicines, even those not prescribed. Unless your doctor tells you otherwise, you/your child should continue to take all prescribed medicines as usual.
If you or your child already has a poor immune system or is already taking medicines that reduce the body's immunity to infections, Rabipur can still be given but you/your child may not be as well protected as other people. In this case, your/your child's doctor may decide to carry out blood tests after the vaccine administration, to check if the body has produced enough antibodies to the virus. If necessary, you/your child will be given extra doses of the vaccine (see section 3 of this leaflet).
You/Your child may also need to be given an injection of antibodies against rabies (called "rabies immunoglobulin")
if you or your child have not been fully vaccinated against rabies and it is very likely that you or your child has been infected with the virus.
# #
If so, the rabies immunoglobulin injection (given only once and usually with the first dose of the vaccine) and the vaccine will be given
in different parts of the body. Usually, as much as possible of the rabies immunoglobulin is injected into the area of the body that came into contact with the animal. Any remaining immunoglobulin is given at a separate injection site.
Pregnancy and breast-feeding
If you are pregnant or breastfeeding, if you think you may be pregnant, or are planning to have a baby, you should still be given rabies vaccine if you have had, or are likely to have had, contact with the virus.
You can also be vaccinated with Rabipur during pregnancy or while breastfeeding and before exposure to the virus, if the risk of contact with the virus is thought to be considerable. In this instance, your doctor will discuss the risks of vaccination and rabies infection with you and advice on the best timing of Rabipur vaccination.
Driving and using machines:
It is not known whether the vaccine has an effect on your ability to drive or use machines. However, some of the adverse effects described in section 4 of this leaflet may affect the ability to drive and use machines.
Rabipur contains:
Less than 23 mg of sodium per dose, and is therefore essentially 'sodium- free'.
3. HOW TO USE RABIPUR
Rabipur will be given to you/your child by a doctor or nurse who has been trained to give vaccines. Treatment that may be needed to manage very serious types of allergic reactions that can occur after receipt of the vaccine should be available (see section 4 of this leaflet). The vaccine should be given to you/your child in a clinic or surgery that has the necessary equipment to treat these reactions.
Instructions intended for doctors and medical personnel for reconstituting the vaccine can be found at the end of this leaflet.
The recommended dose for adults and children of any age is one millilitre (1.0 ml) per injection.
Your doctor will decide how many doses you/your child should receive; this will depend on whether you/ your child are/is being given Rabipur before or after any possible contact with the virus.
The vaccine is given as an injection into a muscle (usually in the upper arm, or in small children, into the muscle of the thigh). The vaccine should not be given into the buttocks, under the skin or into a blood vessel.
BEFORE ANY POSSIBLE CONTACT WITH THE VIRUS
If you have/your child has never had any rabies vaccine before, you/your child will need to have overall 3 doses on days 0, 7 and 21 (or 28).
If you/your child miss an appointment for an injection, you should arrange to have it as soon as possible after the due date.
The need for boosters depends on the risk of contact with rabies virus. Your doctor will consult the official recommendations on rabies vaccination and will tell you when a booster is needed.
If you are at continuous high risk of infection, your doctor may also ask you to have regular blood tests to measure the amount of antibody against rabies in your blood so that boosters can be given as soon as needed. Experience shows that booster doses are generally required every 2-5 years.
AFTER SUSPECTED OR PROVEN CONTACT WITH THE VIRUS Vaccinated people
If you have/your child has already been fully vaccinated against rabies and/or have received boosters, and have been in contact with a rabid or suspected rabid animal, you/ your child usually need 2 more doses of vaccine (1.0 ml each). The first dose is given as soon as possible after the contact, and the second is given 3 days later.
Unvaccinated people
If you/your child have/has not been vaccinated before or received inadequate basic immunization, either 4 or 5 doses (1.0 ml each) will be given.
• If an immunization schedule of 4 doses is used, the first 2 vaccine doses are given as soon as possible after the contact on day 0 and then single doses are given 7 and 21 days after the first dose.
• If an immunization schedule of 5 doses is used, the first vaccine dose is given as soon as possible after the contact on day 0 and the others are given on days 3, 7, 14 and 28 after the first dose.
After any possible contact with rabies virus, your doctor will consider the risk of infection according to the type of contact you have/your child has had. For example, if you have been bitten or scratched by an animal that could have the virus, or have been in contact with bats, you are at much greater risk of rabies infection than someone who has been licked but has no break in the skin.
When vaccination is necessary, the first dose will be given as soon as possible after the contact and the wounds will be treated as follow:
• Thorough flushing and washing of the wound with soap and water
• Applying an antiseptic solution to the wound
• If soap or antiseptic are not available, the wound should be thoroughly and extensively washed with water People with a compromised immune system (poor immunity to infection)
If you have/your child has an increased risk of rabies infection because your immune system is not working properly, you/your child will need five or six doses (each of 1.0 ml) of rabies vaccine after contact with a rabid or suspected rabid animal. Vaccination is given in combination with local treatment of the wound and rabies immunoglobulin.
If six doses are used, the first two are given as soon as possible after the contact, and then single doses are given on days 3, 7, 14 and 28 after the first dose.
If five doses are used, the first dose is given as soon as possible after the contact, and the others are given on days 3, 7, 14 and 28 after the first dose.
It may also be necessary for you/your child to have blood tests to measure the amount of antibody to rabies virus in your/your child's blood so that extra doses of vaccine can be given if needed. Your doctor will explain what needs to be done and will tell you when to attend for extra tests or doses.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious allergic reactions involving the whole body, sometimes associated with shock (dangerously low blood pressure)* can occur following Rabipur vaccination. Appropriate medical treatment and supervision should always be readily available in case of a rare severe allergic reaction to the vaccine. Talk to a doctor straight away if they happen.
The most common side effects reported with the use of Rabipur were pain at the injection site, mainly pain due to the injection, or hardness of the skin at the site of injection. These reactions are very common (occurring in more than 1 in 10 persons).
Most injection site reactions were not severe and resolved within 24 to 48 hours after injection.
Other side effects include:
Very common (these may affect more than 1 in 10 people)
Headache
Dizziness
Rash
General discomfort (malaise)
Fatigue,
Weakness (asthenia)
Fever
Common (these may affect up to 1 in 10 people)
Swollen glands (lymphadenopathy)
Decreased appetite Nausea Vomiting Diarrhoea
Stomach pain/discomfort Hives (urticaria)
Muscles pain
Joint pain (myalgia, arthralgia)
Rare (these may affect up to 1 in 1,000 people)
Allergic reactions (hypersensitivity)
Pins and needles or tingling sensations (paraesthesia)
Sweating (hyperhidrosis)
Chills
Very rare (these may affect up to 1 in 10,000 people)
Inflammation of the brain, nerve disturbances that can cause weakness, inability to move or loss of feeling in some parts of the body* Fainting, unsteadiness with dizziness*
Serious allergic reaction which causes swelling of the face or throat (Angioedema)*
*Adverse reactions from spontaneous reporting Additional side effects in children
Frequency, type and severity of adverse reactions in children are expected to be the same as in adults.
Reporting of side effects
If you or your child gets any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE RABIPUR
Keep this vaccine out of the sight and reach of children.
Store protected from light in a refrigerator (at 2°C to 8°C). Do not freeze.
Do not use this vaccine after the expiry date which is stated on the outer carton. The expiry date refers to the last day of the month.
Do not throw away any vaccine via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION What Rabipur contains
The active substance in the vaccine is rabies virus (inactivated, strain Flury LEP) > 2.5 IU. This has been produced on purified chick embryo cells (PCEC).
The other ingredients are: trometamol, sodium chloride, disodium edetate, potassium-L-glutamate, polygeline, sucrose and water for injections.
What Rabipur looks like and contents of the pack
Rabipur is a white freeze-dried powder which, when reconstituted with the clear colourless solvent, becomes a clear colourless solution.
Rabipur is supplied in packs containing 1 vial of the powder, 1 disposable pre-filled syringe of sterile diluent with 1 small orange needle for injection and one long green needle for reconstitution.
Marketing Authorisation Holder and Manufacturer
Novartis Vaccines and Diagnostics GmbH Emil-von-Behring-Str. 76 35041 Marburg Germany
This medicinal product is authorised in the Member States of the EEA under the following names:
|
Austria |
Rabipur |
Germany |
Rabipur |
|
Belgium |
Rabipur |
Ireland |
Rabipur |
|
Bulgaria |
Rabipur |
Italy |
Rabipur |
|
Croatia |
Rabipur |
Luxembourg |
Rabipur |
|
Czech Republic |
Rabipur |
Netherlands |
Rabipur |
|
Denmark |
Rabipur |
Norway |
Rabipur |
|
France |
Rabipur |
Poland |
Rabipur |
|
Portugal |
Rabipur |
Sweden |
Rabipur |
|
Spain |
Rabipur |
United Kingdom |
Rabipur |
This leaflet was last approved in May 2015 Other sources of information
The following information is intended for healthcare professionals only:
Instruction for use of Rabipur disposable pre-filled syringe Pre-filled syringe
©
Gray Tip Cap
Q
Plastic

0 9® White
Cap Syringe Texured Syringe
Tip Holding Ring
©
Needle
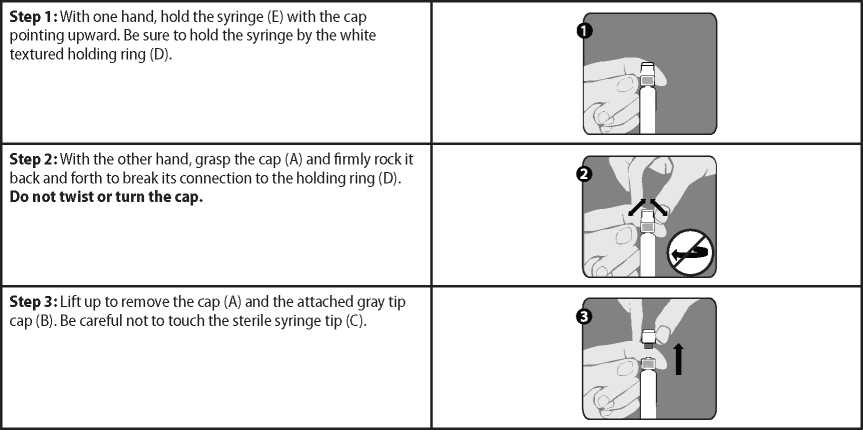
Needle application (these instructions apply to both the green and the orange needles):
|
Step 1: Twist to remove the cap from the green reconstitution needle. Do not remove the plastic cover (G). This needle is the longer of the two needles. |
1 |
ii v-y | |
|
Step 2: With one hand, firmly hold syringe (E) by white textured holding ring (D). With your other hand, insert needle (F) and twist clockwise until it locks into place. Once needle is locked, remove its plastic cover (G). The syringe is now ready for use. |
1 |
MKNq) x L__1_!_ |
Instructions for reconstituting Rabipur with the use of pre-filled syringe:
The vaccine should be visually inspected both before and after reconstitution for any foreign particulate matter and or change in physical appearance. The vaccine must not be used if any change in the appearance of the vaccine has taken place.A clear colorless solution results after reconstitution of the white freeze-dried powder with the clear and colorless solvent of the syringe.
The powder for solution should be reconstituted using the solvent for solution supplied and carefully agitated prior to injection.
The reconstituted vaccine should be used immediately.
The vial of vaccine contains negative pressure. After reconstitution of the vaccine, it is recommended to unscrew the syringe from the needle to eliminate the negative pressure. After that, the vaccine can be easily withdrawn from the vial. It is not recommended to induce excess pressure, since over-pressurization will create the problems in withdrawing the proper amount of the vaccine.
After completing the reconstitution of the vaccine, remove the cap from the orange administration needle (as explained in step 1 for the green needle) and replace the green reconstitution needle with the orange administration needle or other appropriate needle.
lb NOVARTIS
MCK14-137 VACCINES
MCK14-137 GB Rabipur Leaflet- Kit Rosia 301480.indd 2 31/03/2016 14:56:37