Rabipur Powder And Solvent For Solution For Injection In Pre-Filled Syringe
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Rabipur
Powder and solvent for solution for injection in pre-filled syringe. Rabies vaccine, inactivated.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
After reconstitution, 1 vial (1.0 ml) contains:
Rabies virus* (Inactivated, strain Flury LEP)...........................> 2.5 IU
* produced on purified chick embryo cells (PCEC)
This vaccine contains residues of polygeline, chicken proteins (e.g., ovalbumin), human serum albumin, and may contain traces of neomycin, chlortetracycline and amphotericin B. See sections 4.3 and 4.4.
For excipients see section 6.1.
3 PHARMACEUTICAL FORM
Powder and solvent for solution for injection in pre-filled syringe. The powder is white.
The solvent is clear and colourless.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Rabipur is indicated for active immunization against rabies in individuals of all ages.
This includes pre-exposure prophylaxis (i.e. before possible risk of exposure to rabies), in both primary series and booster dose, and post-exposure prophylaxis (i.e. after suspected or proven exposure to rabies).
Rabipur is to be used on the basis of official recommendations.
4.2 Posology and method of administration
Posology
Dosage in adults and children
The recommended single intramuscular (IM) dose is 1.0 ml in individuals of all ages. Pre-exposure prophylaxis (PrEP)
Primary immunization
In previously unvaccinated individuals, an initial course of pre-exposure prophylaxis consists of three doses (each of 1.0 ml) administered IM on days 0, 7, and 21 (or 28).
Booster doses
The individual IM booster dose is 1.0 ml.
Rabipur may be used for booster vaccination after prior immunization with a human diploid cell rabies vaccine (HDCV).
The need of intermittent serological testing for the presence of antibody > 0.5 IU/ml and the administration of booster doses should be assessed in accordance with official recommendations.
Experience shows that reinforcing doses are generally required every 2-5 year.
Post-exposure prophylaxis (PEP)
Post-exposure prophylaxis consists of:
• local treatment of the wound as soon as possible after exposure,
• a course of rabies vaccine and
• administration of rabies immunoglobulin, if indicated
The indication for post-exposure prophylaxis depends on the type of contact with the suspected rabid animal, as provided in Table 1, Recommended post-exposure prophylaxis according to type of exposure. Post-exposure immunisation should begin as soon as possible after exposure.
Table 1: Recommended post-exposure prophylaxis according to type of exposure
Category of Type of exposure to a exposure domestic or wild a) animal
suspected or confirmed to be rabid, or animal unavailable for testing
Recommended post-exposure prophylaxis
|
I |
Touching or feeding animals Licks on intact skin Contact of intact skin with secretions or excretions of a rabid animal or human case |
None, if reliable case history is available. |
|
II |
Nibbling of uncovered skin Minor scratches or abrasions without bleeding |
Administer vaccine immediately b) Stop treatment if animal remains healthy throughout an observation period of 10 days c) or is proven to be negative for rabies by a reliable laboratory using appropriate diagnostic techniques. |
|
III |
Single or multiple transdermal bites d) or scratches, licks on broken skin. Contamination of mucous membrane with saliva (i.e. licks). , Exposure to bats e). |
Administer rabies vaccine immediately, and rabies immunoglobulin, preferably as soon as possible after initiation of post-exposure prophylaxis. Rabies immunoglobulin can be injected up to 7 days after first vaccine dose administration. Stop treatment if animal remains healthy throughout an observation period of 10 days or is proven to be negative for rabies by reliable laboratory using appropriate diagnostic techniques |
a) Exposure to rodents, rabbits or hares does not routinely require rabies post-exposure prophylaxis.
b) If an apparently healthy dog or cat in, or from a low-risk area is placed under observation, treatment may be delayed.
c) This observation period applies only to dogs and cats. Except for threatened or endangered species, other domestic and wild animals suspected of being rabid should be euthanized and their tissues examined for the presence of rabies antigen by appropriate laboratory techniques.
d) Bites especially on the head, neck, face, hands and genitals are category III exposures because of the rich innervation of these areas.
e) Post-exposure prophylaxis should be considered when contact between a human and a bat has occurred, unless the exposed person can rule out a bite or scratch or exposure of a mucous membrane.
Post-exposure prophylaxis of previously unvaccinated individuals
• 5 dose Essen regimen (1-1-1-1-1): one 1.0 ml IM injection on each of days 0, 3, 7, 14
and 28
• 4 dose Zagreb regimen (2-1-1): two 1.0 ml IM injections on day 0 (one in each of the
two deltoids or thigh sites) followed by one 1.0 ml IM injection on each of days 7 and 21.
Post-exposure prophylaxis in previously vaccinated individuals
In previously vaccinated individuals, post-exposure prophylaxis consists of two doses (each of 1.0 ml) administered IM on days 0, and 3. Rabies immunoglobulin is not indicated in such cases.
Paediatric patients
Paediatric individuals receive the same 1.0 ml IM dose as adults.
Geriatric patients
Geriatric individuals receive the same 1.0 ml IM dose as adults.
Immunocompromised individuals
In immunocompromised individuals, a complete series of 5 doses according to the Essen (1-1-1-1-1) on days 0, 3, 7, 14 and 28) regimen in combination with comprehensive wound management and local infiltration of rabies immunoglobulin is required for individuals with category II and III exposure.
Alternatively, two doses of vaccine may be given on day 0, that is, a single dose of 1.0 ml vaccine should be injected into the right deltoid and another single dose into the left deltoid muscle. In small children, one dose should be given into the anterolateral region of each thigh. This would result in a total of 6 doses (2-1-1-1-1 on days 0, 3, 7, 14 and 28).
When feasible, the rabies virus neutralising antibody response should be measured 2 to 4 weeks (preferably on day 14) following the start of vaccination to assess the possible need for an additional dose of the vaccine. Immunosuppressive agents should not be administered during postexposure therapy unless essential for the treatment of other conditions (see section 4.5).
Method of administration
For adults and children > 2 years of age, the vaccine should be administered intramuscular into the deltoid; for children < 2 years, the anterolateral area of the thigh is recommended.
The vaccine must not be given by intravascular injection, see section 4.4.
Rabies vaccine must not be given by intra-gluteal injection or subcutaneously, see section 4.4.
For instructions on reconstitution of the vaccine before administration, see section 6.6.
4.3 Contraindications
Pre-exposure prophylaxis (PrEP)
History of a severe hypersensitivity to the active substance, any of the excipients listed in section 6.1 or residues in section 2
Individuals with acute diseases requiring treatment should not be vaccinated until at least 2 weeks after recovery. Minor infections are not a contraindication to vaccination.
Post-exposure prophylaxis (PEP)
In view of the almost invariably fatal outcome of rabies, there is no contraindication to post-exposure prophylaxis, including pregnancy.
4.4 Special warnings and precautions for use
Reports of anaphylactic reactions including anaphylactic shock have occurred following Rabipur vaccination. As with all injectable vaccines, appropriate medical treatment and supervision should always be readily available in case of a rare anaphylactic event following the administration of the vaccine.
Patients considered to be at risk of a severe hypersensitivity reaction to the vaccine or any of the vaccine components should receive an alternative rabies vaccine if a suitable product is available.
Encephalitis and Guillain-Barre syndrome have been reported to be temporally associated with the use of Rabipur (see also section 4.8). The use of corticosteroids to treat adverse reactions such as these may inhibit the development of immunity to rabies (see section 4.5). A patient's risk of developing rabies must be carefully considered, before deciding to discontinue immunization.
Unintentional intravascular injection may result in systemic reactions, including shock. Do not inject intravascularly. The vaccine must not be mixed in the same syringe with other medicinal products. If rabies immunoglobulin is indicated in addition to Rabipur vaccine, then it must be administered at an anatomical site distant to the vaccination (see section 4.5).
Anxiety-related reactions, including vasovagal reactions (syncope), hyperventilation or stress-related reactions, may occur in association with vaccination as a psychogenic response to the needle injection (see section 4.8). It is important that procedures are in place to avoid injury from fainting.
Rabies vaccine must not be given by intra-gluteal injection or subcutaneously, as the induction of an adequate immune response may be less reliable.
4.5 Interaction with other medicinal products and other forms of interaction
Immunosuppressive agents can interfere with the development of an adequate response to the rabies vaccine. Therefore, it is recommended that serological responses should be monitored in such subjects, and additional doses administered as necessary (see section 4.2).
All of the rabies immunoglobulin, or as much as anatomically possible (but avoiding possible compartment syndrome), should be administered into or around the wound site or sites. The remaining immunoglobulin, if any, should be injected intramuscularly at a site distant from the site of vaccine administration to avoid possible interference with simultaneously administered rabies vaccine.
Other inactivated vaccines may be given at the same time as Rabipur. Concomitant vaccines should always be administrated at separate injection sites and preferably contralateral limbs.
4.6 Fertility, pregnancy and lactation
Pregnancy
No cases of harm attributable to use of Rabipur during pregnancy have been observed.
Rabipur may be administered to pregnant women when post-exposure prophylaxis is required.
The vaccine may also be used for pre-exposure prophylaxis during pregnancy if it is considered that the potential benefit outweighs any possible risk to the foetus.
Breastfeeding
While it is not known whether Rabipur enters breast milk, no risk to the breastfeeding infant has been identified. Rabipur may be administered to breastfeeding women when post-exposure prophylaxis is required.
The vaccine may also be used for pre-exposure prophylaxis in breastfeeding women if it is considered that the potential benefit outweighs any possible risk to the infant.
Fertility
Non clinical reproductive and developmental toxicity studies have not been performed.
4.7 Effects on ability to drive and use machines
No studies have been carried out with Rabipur to assess the effect on the ability to drive or use machines (see also section 4.8).
Some of the adverse effects described in section 4.8, may affect the ability to drive and use machines.
4.8 Undesirable effects
Summary of the safety profile
Anaphylactic reactions including anaphylactic shock that are very rare but clinically severe, and potentially lethal, systemic allergic reactions, can occur following Rabipur vaccination. Anaphylaxis was not reported during clinical trials of Rabipur.
The most commonly reported solicited adverse reactions were injection site pain (3085%, mainly pain due to injection) or injection site induration (15-35%). Most injection site reactions were not severe and resolved within 24 to 48 hours after injection.
Mild allergic reactions to Rabipur (i.e. Hypersensitivity), including rashes and urticaria may occur after vaccination. Rashes may occur in more than 1 in 10 people, and urticaria may occur in between 1 in 100 and 1 in 10 people. These reactions are usually mild in nature and typically resolve within a few days.
A small number of people have reported symptoms of encephalitis and Guillain-Barre Syndrome following Rabipur vaccination.
Tabulated list of adverse reactions
The following vaccine-related adverse reactions were reported in clinical studies and during post-marketing surveillance. During post-marketing experience, the adverse events are reported voluntarily from a population of uncertain size wherein an estimate of frequency cannot be made. Hence, these events have been chosen for inclusion due to their seriousness, frequency of reporting, causal relationship to Rabipur, or a combination of these factors.
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. In addition, the corresponding frequency category using the following convention (CIOMS III) is also provided for each adverse reaction: very common (>1/10); common (>1/100, <1/10); uncommon (>1/1,000, <1/100); rare (>1/10,000, <1/1,000) and very rare (<1/10,000)
|
System Organ Class |
Frequency |
Adverse events |
|
Blood and lymphatic system disorders |
Common |
Lymphadenopathy |
|
Immune system disorders |
Rare |
Hypersensitivity |
|
Very rare |
Anaphylaxis including anaphylactic shock* | |
|
Metabolism and nutrition disorder |
Common |
Decreased appetite |
|
Nervous system disorders |
Very common |
Headache, Dizziness |
|
Rare |
Paraesthesia | |
|
Very rare |
Encephalitis*, Guillain-Barre syndrome*, Presyncope*, Syncope*, Vertigo* | |
|
Gastrointestinal disorders |
Common |
Nausea, Vomiting, Diarrhoea, Abdominal |
|
pain/ discomfort | ||
|
Skin and subcutaneous tissue disorders |
Very common |
Rash |
|
Common |
Urticaria | |
|
Rare |
Hyperhidrosis (sweating) | |
|
Very rare |
Angioedema* | |
|
Musculoskeletal and connective tissue disorders |
Common |
Myalgia, Arthralgia |
|
General disorder and administration site conditions |
Very common |
Injection site reactions, Malaise, Fatigue, Asthenia, Fever |
|
Rare |
Chills |
*Adverse reactions from spontaneous reporting
Description of selected adverse reactions
Headache and dizziness were majorly reported during the post-marketing experience where these remained of transient nature and resolved without any intervention.
Adverse event rash followed the same post-marketing surveillance experience where in majority of cases was reported with limited details with no description provided; however, few cases for rash were reported in association with potential hypersensitivity reaction.
Injection site reactions were reported variably and primarily included adverse event terms of injection site pain/ discomfort/ induration/ swelling/ erythema and oedema. All these had a favorable outcome even if the treatment was started. None of these qualified as injection-site cellulitis, and hence remained of transient nature. In general, injection site reactions were commonly reported in clinical trials with Rabipur and the post marketing data supports this finding.
For the treatment to hypersensitivity (allergic) reactions, reference is made to section 4.4.
Once initiated, rabies post-exposure prophylaxis should not be interrupted or discontinued because of local or mild systemic adverse reactions to rabies vaccine.
Paediatric population
Frequency, type and severity of adverse reactions in children are expected to be the same as in adults.
Other special populations
Rabipur has never been studied exclusively in any specific population group such as elderly, patients with renal impairment, patients with hepatic impairment, and elderly individuals patients with other diseases or a specific genotype, because the risk factors remains the same across all populations. However, these special populations were not specifically excluded from Rabipur clinical trials and no clinically relevant
differences (i.e. in nature, frequency, seriousness or reversibility of adverse reactions, or need for monitoring) have been specifically observed.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No symptoms of overdose are known.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
ATC code: J07B G01
The minimum rabies virus antibody titre recommended as being proof of an adequate immune response after vaccination is > 0.5 IU/ml concentration as specified by the WHO. In healthy vaccinees, this level should be achieved in most individuals by Day 14 of a postexposure regimen, with or without simultaneous administration of RIG and irrespective of age.
Pre-exposure prophylaxis
In clinical trials with previously unimmunised subjects, almost all subjects achieve an adequate immune response (RVNAs > 0.5 IU/ml) 3 to 4 weeks after the end of a primary series of three injections of Rabipur when given according to the recommended schedule by the intramuscular route. Persistence of adequate immune response (RVNAs > 0.5 IU/ml) for up to 2 years after immunization with Rabipur without additional booster has been found in clinical studies. As antibody concentrations slowly decrease, booster doses may be required to maintain antibody levels above 0.5 IU/ml. The need for and timing of boosting should be assessed on a case by case basis, taking into account official guidance (see also section 4.2).
In a clinical trial, a booster dose of Rabipur administered 1 year after primary immunisation elicited a 10-fold or higher increase in Geometric Mean Concentrations (GMCs) by day 30. It has also been demonstrated that individuals who had previously been immunised with Human Diploid Cell Vaccine (HDCV) developed a rapid anamnestic response when boosted with Rabipur.
Post-exposure prophylaxis
In clinical studies Rabipur elicited adequate neutralising antibodies (> 0.5 IU/ml) in almost all subjects by day 14 or 30, when administered according to the WHO-recommended 5- dose* (day 0, 3, 7, 14, 28; 1.0 ml each, intramuscular) Essen regimen or to the WHO recommended 4-dose (day 0 [2 doses], 7, 21; 1.0 ml each, intramuscular) Zagreb regimen.
* Former WHO recommended Essen regimen consisted of 6 doses (day 0, 3, 7, 14, 28, 90; 1.0 ml each, intramuscularly).
Concomitant administration of Human Rabies Immunoglobulin (HRIG) with the first dose of rabies vaccine caused a slight decrease in GMCs (Essen regimen). However, this was not considered to be clinically relevant nor statistically significant.
5.2 Pharmacokinetic properties
Not applicable
5.3 Preclinical safety data
Preclinical data including single-dose, repeated dose and local tolerance studies revealed no unexpected findings and no target organ toxicity. No genotoxicity, carcinogenicity and reproductive toxicity studies have been performed.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Powder:
Trometamol
Sodium chloride
Disodium edetate
Potassium-L-glutamate
Polygeline
Sucrose
Solvent:
Water for injection
6.2 Incompatibilities
In the absence of compatibility studies, Rabipur must not be mixed in the same syringe with other medicinal products.
6.3 Shelf life
48 months
After reconstitution the vaccine is to be used immediately.
6.4 Special precautions for storage
Store in a refrigerator (2°C - 8°C). Do not freeze.
Keep the vials in the outer carton in order to protect from light.
For storage conditions after reconstitution of the medicinal product, see section 6.3
The vaccine may not be used after the expiration date given on package and container.
6.5 Nature and contents of container
Package with:
1 vial (type I glass) of freeze-dried vaccine with stopper (chlorobutyl)
1 disposable pre-filled syringe (type I glass) of Sterile Diluent for reconstitution (1 mL) with plunger-stopper (bromobutyl) without needle and with a tip-cap (bromobutyl). 1 small orange needle for injection (25 gauge, 27 mm) and one long green needle for reconstitution (21 gauge, 40 mm)
6.6 Special precautions for disposal
Instruction for Use of Rabipur disposable pre-filled syringe:
Pre-filled syringe
Plastic
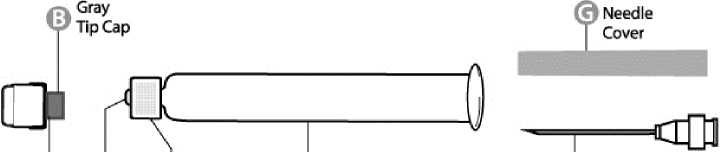
O (9 ©White @ 6
Cap Syringe Texured Syringe Needle
Tip Holding Ring
Step 1: With one hand, hold the syringe (E) with the cap pointing upward. Be sure to hold the syringe by the white textured holding ring (D).
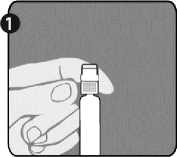
Step 2: With the other hand, grasp the cap (A) and firmly rock it back and forth to break its connection to the holding ring (D). Do not twist or turn the cap.
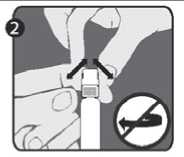
Step 3: Lift up to remove the cap (A) and the attached gray tip cap (B). Be careful not to touch the sterile syringe tip (C).
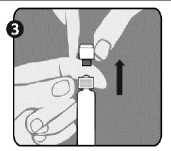
Needle application (these instructions apply to both the green and the orange needles):
Step 1: Twist to remove the cap from the green reconstitution needle. Do not remove the plastic cover (G). This needle is the longer of the two needles.
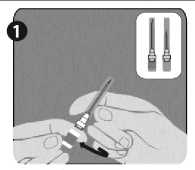
Step 2: With one hand, firmly hold syringe (E) by white textured holding ring (D). With your other hand, insert needle (F) and twist clockwise until it locks into place. Once needle is locked, remove its plastic cover (G)
The syringe is now ready for use.
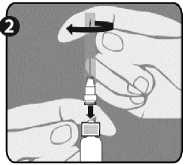
Instructions for reconstituting Rabipur with the use of pre-filled syringe:
The vaccine should be visually inspected both before and after reconstitution for any foreign particulate matter and or change in physical appearance. The vaccine must not be used if any change in the appearance of the vaccine has taken place.
A clear colourless solution results after reconstitution of the white freeze-dried powder with the clear and colourless solvent of the syringe.
The powder for solution should be reconstituted using the solvent for solution supplied and carefully agitated prior to injection. The reconstituted vaccine should be used immediately.
During manufacturing, the vial is sealed under vacuum. Therefore to prevent problems in withdrawing the reconstituted vaccine from the vial after reconstitution of the vaccine, it is recommended to unscrew the syringe from the needle to eliminate the negative pressure. After that, the vaccine can be easily withdrawn from the vial. It
is not recommended to induce excess pressure, since over-pressurization will create the problems in withdrawing the proper amount of the vaccine.
After completing the reconstitution of the vaccine, remove the cap from the orange administration needle (as explained in step 1 for the green needle) and replace the green reconstitution needle with the orange administration needle or other appropriate needle.
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Novartis Vaccines and Diagnostics GmbH P.O. Box 16 30 35006 Marburg Germany
8 MARKETING AUTHORISATION NUMBER(S)
PL 16033/0010
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
06/04/2016
10 DATE OF REVISION OF THE TEXT
06/04/2016