Rapitil 2% W/V Eye Drops
Out of date information, search anotherRef: 1277/200114/1/F
Rapitil ® 2% w/v Eye Drops
(nedocromil sodium)
Patient Information Leaflet
Read all of this leaflet carefully before you start taking this medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Your medicine is called Rapitil 2% w/v Eye Drops, but will be referred to as Rapitil through out this leaflet.
In this leaflet:
What Rapitil is and what it is used for [4 Before you use Rapitil 3 How to use Rapitil [4 Possible side effects i4 How to store Rapitil ^4 Further information
What Rapitil is and what it is used for
What Rapitil Eye Drops are
Rapitil 2% Eye Drops contain a medicine called nedocromil sodium. This belongs to a group of medicines called anti-allergics.
How Rapitil Eye Drops work
It works by stopping the release of the natural substances in your eyes that can lead to an allergic reaction.
What Rapitil Eye Drops can be used for
Stopping, treating and relieving the symptoms of ‘allergic conjunctivitis' (inflammation of parts of the eye). Rapitil Eye Drops can be used for three types of allergic conjunctivitis:
• Perennial allergic conjunctivitis' in adults only:
This type of allergy can happen at any time of the year and is often caused by animal fur or house dust mites. Signs include itchy, watery, red or inflamed eyes and puffy eyelids.
• ‘Seasonal allergic conjunctivitis' in adults and children: This type of allergy, such as hay fever, can be caused by different pollens in different seasons of the year. Signs include itchy, watery, red or inflamed eyes and puffy eyelids.
• ‘Vernal kerato conjunctivitis' in adults and children: This is a more severe type of allergy. Signs include bumps inside the upper eyelid, sensitivity to light and severe itching.
[4 Before you use Rapitil
Do not use this medicine and tell your doctor or pharmacist if:
• You are allergic (hypersensitive) to nedocromil sodium, or any of the other ingredients of Rapitil Eye Drops (listed in Section 6 below) Signs of an allergic reaction include: a rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue or around the eyes.
Do not use this medicine if the above applies to you. If you are not sure, talk to your doctor or pharmacist before using Rapitil Eye Drops.
Take special care with Rapitil Eye Drops if:
• You wear soft contact lenses. You should not wear soft contact lenses while using these drops.
If you are not sure if this applies to you, talk to your doctor or pharmacist before using Rapitil Eye Drops.
Pregnancy and breast-feeding
Talk to your doctor before using this medicine if you are pregnant, might become pregnant or think you may be pregnant.
If you are breast-feeding or planning to breast-feed, talk to your doctor or pharmacist before taking or using any medicine.
Driving and using machines
Your eyesight may be affected after using this medicine. If this happens, do not drive or use any tools or machines until you can see clearly.
Important information about some of the ingredients of Rapitil Eye Drops
Rapitil Eye Drops contain benzalkonium chloride. This may cause your eyes to become irritated. It may also change the colour of your contact lenses.
r4 How to use Rapitil
Always use Rapitil Eye Drops exactly as your doctor
or pharmacist has told you. You should check with
your doctor or pharmacist if you are not sure.
Using this medicine
• If you wear hard or gas-permeable contact lenses, you should take them out while you use the eye drops. You should wait 15 minutes before you put the contact lenses back in.
• If you need to use more than one type of eye medicine, the medicines must be used at least 5 minutes apart. Eye ointments should be used last.
• If you feel the effect of your medicine is too weak or too strong, do not change the dose yourself, but ask your doctor or pharmacist.
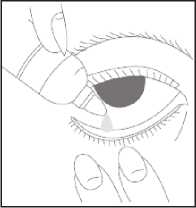
How to use this medicine
• Wash your hands
• Remove the cap from the bottle
• Tilt your head back
• Squeeze one or two drops inside the lower lid without touching your eye
• Close your eye
• Wipe away any excess liquid from the eyes with a clean tissue
• Always put the cap back on the bottle as soon as you have used it
• Repeat in the other eye as needed.
How much to use
Adults, Elderly and Children aged 6 years and over
Seasonal allergic conjunctivitis:
• One drop into each eye twice each day
• This can be increased to four times each day if needed
• Do not use for more than 12 weeks Vernal kerato-conjunctivitis
• One drop into each eye four times each day
Adults and the Elderly only Perennial allergic conjunctivitis:
• One drop into each eye twice each day
• This can be increased to four times each day if needed
• Use the drops regularly Children under 6 years
This medicine is not recommended for children aged less than 6 years.
If you take more Rapitil Eye Drops than you should
If you use more Rapitil than you should, talk to your doctor or go to a hospital straight away. Remember to take any medicine that is left with you so the doctor knows what you have taken.
If you forget to use Rapitil Eye Drops
If you forget a dose, use your drops as soon as you remember. However, if it is nearly time for your next dose, skip the missed dose. Do not use a double dose to make up for a forgotten dose.
If you stop using Rapitil Eye Drops
Keep using the eye drops for as long as your doctor or pharmacist advises you. If you stop using Rapitil Eye drops too early, your allergy symptoms may come back.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
[4) Possible side effects
Like all medicines, Rapitil Eye Drops can cause side effects, although not everybody gets them.
Stop using Rapitil Eye Drops and see a doctor as soon as possible if:
• The itching, redness or swelling gets worse. You may be allergic to these drops
Talk to your doctor or pharmacist if any of the side effects gets serious or lasts longer than a few days, or if you notice any side effects not listed in this leaflet:
Common (affects 1 to 10 people in a 100)
• Stinging or burning in your eyes or blurring of eyesight. This should only last for a short time and occurs immediately after using the eye drops
• Change in the way things taste Uncommon (affects 1 to 10 people in a 1,000)
• Mild eye irritation
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
Eye drops should be kept in the original carton and not stored above 25°C and out of direct sunlight.
KEEP OUT OF THE SIGHT AND REACH OF CHILDREN
Avoid contamination of the contents by replacing the cap securely after use.
You must not use the eye drops after the expiry date. This is given in two places on the end flap of the carton and on the bottle label. In both places it is given as EXP followed by the month and year. The eye drops should not be used after the end of this month. Discard the eye drops four weeks after opening the first time.
Remember this medicine is for you. It can only be prescribed by a doctor. Never give your medicine to other people. It may harm them, even if their symptoms are the same as yours. This leaflet does not tell you everything about your medicine. If you have any questions or are not sure about anything, ask your doctor or pharmacist (chemist). He/she will have additional information about this medicine and will be able to advise you.
Further Information
What Rapitil contains
Each bottle contains 5ml of sterile, preserved aqueous solution containing 2% of the active ingredient nedocromil sodium. Your medicine also contains the following inactive ingredients Benzalkonium chloride, sodium chloride, disodium edetate, and purified water.
The active ingredient belongs to a group of medicines known as anti-allergic / anti-inflammatory agents.
What Rapitil looks like and contents of the pack
This medicine is presented as a 5 ml sterile solution. Each ml of solution contains 2% nedocromil sodium, in a dropper bottle for administration to the eye.
Manufacturer and Licence Holder
The eye drops are manufactured by Sanofi Winthrop Industrie, Boulevard Industrial, Le Trait F-76580, France and procured from within the EU and repackaged by the Product Licence Holder: Lexon (UK) Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 0RE.
| POM PL 15184/1277
Leaflet revision date: 20/01/14
Rapitil is a registered trademark of Fisons Limited
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Tilavist 2% w/v Eye Drops
(nedocromil sodium)
Patient Information Leaflet
Read all of this leaflet carefully before you start taking this medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Your medicine is called Tilavist 2% Eye Drops, but will be referred to as Tilavist through out this leaflet.
In this leaflet:
What Tilavist is and what it is used for Before you use Tilavist How to use Tilavist Possible side effects [a How to store Tilavist Further information
What Tilavist is and what it is used for
What Tilavist Eye Drops are
Tilavist 2% Eye Drops contain a medicine called nedocromil sodium. This belongs to a group of medicines called anti-allergics.
How Tilavist Eye Drops work
It works by stopping the release of the natural substances in your eyes that can lead to an allergic reaction.
What Tilavist Eye Drops can be used for
Stopping, treating and relieving the symptoms of ‘allergic conjunctivitis' (inflammation of parts of the eye). Tilavist Eye Drops can be used for three types of allergic conjunctivitis:
• Perennial allergic conjunctivitis' in adults only:
This type of allergy can happen at any time of the year and is often caused by animal fur or house dust mites. Signs include itchy, watery, red or inflamed eyes and puffy eyelids.
• ‘Seasonal allergic conjunctivitis' in adults and children: This type of allergy, such as hay fever, can be caused by different pollens in different seasons of the year. Signs include itchy, watery, red or inflamed eyes and puffy eyelids.
• ‘Vernal kerato conjunctivitis' in adults and children: This is a more severe type of allergy. Signs include bumps inside the upper eyelid, sensitivity to light and severe itching.
*2 Before you use Tilavist
Do not use this medicine and tell your doctor or pharmacist if:
• You are allergic (hypersensitive) to nedocromil sodium, or any of the other ingredients of Tilavist Eye Drops (listed in Section 6 below) Signs of an allergic reaction include: a rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue or around the eyes.
Do not use this medicine if the above applies to you. If you are not sure, talk to your doctor or pharmacist before using Tilavist Eye Drops.
Take special care with Tilavist Eye Drops if:
• You wear soft contact lenses. You should not wear soft contact lenses while using these drops.
If you are not sure if this applies to you, talk to your doctor or pharmacist before using Tilavist Eye Drops.
Pregnancy and breast-feeding
Talk to your doctor before using this medicine if you are pregnant, might become pregnant or think you may be pregnant.
If you are breast-feeding or planning to breast-feed, talk to your doctor or pharmacist before taking or using any medicine.
Driving and using machines
Your eyesight may be affected after using this medicine. If this happens, do not drive or use any tools or machines until you can see clearly.
Important information about some of the ingredients of Tilavist Eye Drops
Tilavist Eye Drops contain benzalkonium chloride. This may cause your eyes to become irritated. It may also change the colour of your contact lenses.
How to use Tilavist
Always use Tilavist Eye Drops exactly as your
doctor or pharmacist has told you. You should check
with your doctor or pharmacist if you are not sure.
Using this medicine
• If you wear hard or gas-permeable contact lenses, you should take them out while you use the eye drops. You should wait 15 minutes before you put the contact lenses back in.
• If you need to use more than one type of eye medicine, the medicines must be used at least 5 minutes apart. Eye ointments should be used last.
• If you feel the effect of your medicine is too weak or too strong, do not change the dose yourself, but ask your doctor or pharmacist.
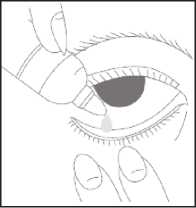
How to use this medicine
• Wash your hands
• Remove the cap from the bottle
• Tilt your head back
• Squeeze one or two drops inside the lower lid without touching your eye
• Close your eye
• Wipe away any excess liquid from the eyes with a clean tissue
• Always put the cap back on the bottle as soon as you have used it
• Repeat in the other eye as needed.
How much to use
Adults, Elderly and Children aged 6 years and over
Seasonal allergic conjunctivitis:
• One drop into each eye twice each day
• This can be increased to four times each day if needed
• Do not use for more than 12 weeks Vernal kerato-conjunctivitis
• One drop into each eye four times each day
Adults and the Elderly only Perennial allergic conjunctivitis:
• One drop into each eye twice each day
• This can be increased to four times each day if needed
• Use the drops regularly Children under 6 years
This medicine is not recommended for children aged less than 6 years.
If you take more Tilavist Eye Drops than you should
If you use more Tilavist than you should, talk to your doctor or go to a hospital straight away. Remember to take any medicine that is left with you so the doctor knows what you have taken.
If you forget to use Tilavist Eye Drops
If you forget a dose, use your drops as soon as you remember. However, if it is nearly time for your next dose, skip the missed dose. Do not use a double dose to make up for a forgotten dose.
If you stop using Tilavist Eye Drops
Keep using the eye drops for as long as your doctor or pharmacist advises you. If you stop using Tilavist Eye drops too early, your allergy symptoms may come back.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Possible side effects
Like all medicines, Tilavist Eye Drops can cause side effects, although not everybody gets them.
Stop using Tilavist Eye Drops and see a doctor as soon as possible if:
• The itching, redness or swelling gets worse. You may be allergic to these drops
Talk to your doctor or pharmacist if any of the side effects gets serious or lasts longer than a few days, or if you notice any side effects not listed in this leaflet:
Common (affects 1 to 10 people in a 100)
• Stinging or burning in your eyes or blurring of eyesight. This should only last for a short time and occurs immediately after using the eye drops
• Change in the way things taste Uncommon (affects 1 to 10 people in a 1,000)
• Mild eye irritation
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
Eye drops should be kept in the original carton and not stored above 25°C and out of direct sunlight.
KEEP OUT OF THE SIGHT AND REACH OF CHILDREN
Avoid contamination of the contents by replacing the cap securely after use.
You must not use the eye drops after the expiry date. This is given in two places on the end flap of the carton and on the bottle label. In both places it is given as EXP followed by the month and year. The eye drops should not be used after the end of this month. Discard the eye drops four weeks after opening the first time.
Remember this medicine is for you. It can only be prescribed by a doctor. Never give your medicine to other people. It may harm them, even if their symptoms are the same as yours. This leaflet does not tell you everything about your medicine. If you have any questions or are not sure about anything, ask your doctor or pharmacist (chemist). He/she will have additional information about this medicine and will be able to advise you.
[6 Further Information
What Tilavist contains
Each bottle contains 5ml of sterile, preserved aqueous solution containing 2% of the active ingredient nedocromil sodium. Your medicine also contains the following inactive ingredients Benzalkonium chloride, sodium chloride, disodium edetate, and purified water.
The active ingredient belongs to a group of medicines known as anti-allergic / anti-inflammatory agents.
What Tilavist looks like and contents of the pack
This medicine is presented as a 5 ml sterile solution. Each ml of solution contains 2% nedocromil sodium, in a dropper bottle for administration to the eye.
Manufacturer and Licence Holder
The eye drops are manufactured by Sanofi Winthrop Industrie, Boulevard Industrial, Le Trait F-76580, France and procured from within the EU and repackaged by the Product Licence Holder: Lexon (UK) Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 0RE.
POM PL 15184/1277 Leaflet revision date: 20/01/14 Tilavist is a registered trademark of Fisons Limited
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Nedocromil Sodium 2% w/v Eye Drops
(nedocromil sodium)
Patient Information Leaflet
Read all of this leaflet carefully before you start taking this medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Your medicine is called Nedocromil Sodium 2% Eye Drops but will be referred to as Nedocromil Sodium through out this leaflet.
In this leaflet:
™ What nedocromil sodium is and what it is used for
^ Before you use nedocromil sodium [3 How to use nedocromil sodium i4 Possible side effects ^ How to store nedocromil sodium ^ Further information
^ What Nedocromil sodium is and what it is used for
What nedocromil sodium Eye Drops are
Nedocromil sodium 2% Eye Drops contain a medicine called nedocromil sodium. This belongs to a group of medicines called anti-allergics.
How Nedocromil sodium Eye Drops work
It works by stopping the release of the natural substances in your eyes that can lead to an allergic reaction.
What Nedocromil sodium Eye Drops can be used for
Stopping, treating and relieving the symptoms of ‘allergic conjunctivitis' (inflammation of parts of the eye). Nedocromil sodium Eye Drops can be used for three types of allergic conjunctivitis:
• Perennial allergic conjunctivitis' in adults only: This type of allergy can happen at any time of the year and is often caused by animal fur or house dust mites. Signs include itchy, watery, red or inflamed eyes and puffy eyelids.
• ‘Seasonal allergic conjunctivitis' in adults and children: This type of allergy, such as hay fever, can be caused by different pollens in different seasons of the year. Signs include itchy, watery, red or inflamed eyes and puffy eyelids.
• ‘Vernal kerato conjunctivitis' in adults and children: This is a more severe type of allergy. Signs include bumps inside the upper eyelid, sensitivity to light and severe itching.
Before you use Nedocromil sodium
Do not use this medicine and tell your doctor or pharmacist if:
• You are allergic (hypersensitive) to nedocromil sodium, or any of the other ingredients of Nedocromil sodium Eye Drops (listed in Section 6 below) Signs of an allergic reaction include: a rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue or around the eyes.
Do not use this medicine if the above applies to you. If you are not sure, talk to your doctor or pharmacist before using Nedocromil sodium Eye Drops.
Take special care with Nedocromil sodium Eye Drops if:
• You wear soft contact lenses. You should not wear soft contact lenses while using these drops.
If you are not sure if this applies to you, talk to your doctor or pharmacist before using Nedocromil sodium Eye Drops.
Pregnancy and breast-feeding
Talk to your doctor before using this medicine if you are pregnant, might become pregnant or think you may be pregnant.
If you are breast-feeding or planning to breast-feed, talk to your doctor or pharmacist before taking or using any medicine.
Driving and using machines
Your eyesight may be affected after using this medicine. If this happens, do not drive or use any tools or machines until you can see clearly.
Important information about some of the ingredients of Nedocromil sodium Eye Drops
Nedocromil sodium Eye Drops contain benzalkonium chloride. This may cause your eyes to become irritated. It may also change the colour of your contact lenses.
How to use Nedocromil sodium
Always use Nedocromil sodium Eye Drops exactly as your doctor or pharmacist has told you. You should check with your doctor or pharmacist if you are not sure.
Using this medicine
• If you wear hard or gas-permeable contact lenses, you should take them out while you use the eye drops. You should wait 15 minutes before you put the contact lenses back in.
• If you need to use more than one type of eye medicine, the medicines must be used at least 5 minutes apart. Eye ointments should be used last.
• If you feel the effect of your medicine is too weak or too strong, do not change the dose yourself, but ask your doctor or
pharmacist.
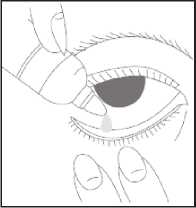
How to use this medicine
• Wash your hands
• Remove the cap from the bottle
• Tilt your head back
• Squeeze one or two drops inside the lower lid without touching your eye
• Close your eye
• Wipe away any excess liquid from the eyes with a clean tissue
• Always put the cap back on the bottle as soon as
you have used it
• Repeat in the other eye as needed.
How much to use
Adults, Elderly and Children aged 6 years and over
Seasonal allergic conjunctivitis:
• One drop into each eye twice each day
• This can be increased to four times each day if needed
• Do not use for more than 12 weeks Vernal kerato-conjunctivitis
• One drop into each eye four times each day
Adults and the Elderly only Perennial allergic conjunctivitis:
• One drop into each eye twice each day
• This can be increased to four times each day if needed
• Use the drops regularly Children under 6 years
This medicine is not recommended for children aged less than 6 years.
If you take more Tilavist Eye Drops than you should
If you use more Tilavist than you should, talk to your doctor or go to a hospital straight away. Remember to take any medicine that is left with you so the doctor knows what you have taken.
If you forget to use Nedocromil sodium Eye Drops
If you forget a dose, use your drops as soon as you remember. However, if it is nearly time for your next dose, skip the missed dose. Do not use a double dose to make up for a forgotten dose.
If you stop using Nedocromil sodium Eye Drops
Keep using the eye drops for as long as your doctor or pharmacist advises you. If you stop using Nedocromil sodium Eye drops too early, your allergy symptoms may come back.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Possible side effects
Like all medicines, Nedocromil sodium Eye Drops can cause side effects, although not everybody gets them.
Stop using Nedocromil sodium Eye Drops and see a doctor as soon as possible if:
• The itching, redness or swelling gets worse. You may be allergic to these drops
Talk to your doctor or pharmacist if any of the side effects gets serious or lasts longer than a few days, or if you notice any side effects not listed in this leaflet:
Common (affects 1 to 10 people in a 100)
• Stinging or burning in your eyes or blurring of eyesight. This should only last for a short time and occurs immediately after using the eye drops
• Change in the way things taste Uncommon (affects 1 to 10 people in a 1,000)
• Mild eye irritation
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
Eye drops should be kept in the original carton and not stored above 25°C and out of direct sunlight.
KEEP OUT OF THE SIGHT AND REACH OF CHILDREN
Avoid contamination of the contents by replacing the cap securely after use.
You must not use the eye drops after the expiry date. This is given in two places on the end flap of the carton and on the bottle label. In both places it is given as EXP followed by the month and year. The eye drops should not be used after the end of this month. Discard the eye drops four weeks after opening the first time.
Remember this medicine is for you. It can only be prescribed by a doctor. Never give your medicine to other people. It may harm them, even if their symptoms are the same as yours. This leaflet does not tell you everything about your medicine. If you have any questions or are not sure about anything, ask your doctor or pharmacist (chemist). He/she will have additional information about this medicine and will be able to advise you.
3 Further Information
What Rapitil contains
Each bottle contains 5ml of sterile, preserved aqueous solution containing 2% of the active ingredient nedocromil sodium. Your medicine also contains the following inactive ingredients Benzalkonium chloride, sodium chloride, disodium edetate, and purified water.
The active ingredient belongs to a group of medicines known as anti-allergic / anti-inflammatory agents.
What Rapitil looks like and contents of the pack
This medicine is presented as a 5 ml sterile solution. Each ml of solution contains 2% nedocromil sodium, in a dropper bottle for administration to the eye.
Manufacturer and Licence Holder
The eye drops are manufactured by Sanofi Winthrop Industrie, Boulevard Industrial, Le Trait F-76580, France and procured from within the EU and repackaged by the Product Licence Holder: Lexon (UK) Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 0RE.
| POM PL 15184/1277 Leaflet revision date: 20/01/14
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Ref: 1277/200114/3/B