Saflutan 15 Micrograms/Ml Eye Drops Solution In Single-Dose Container
Out of date information, search another|
200 | ||
|
^ 14 J |
T~ o v | |

1SAFL2520GB
Driving and using machines
SAFLUTAN has no influence on the ability to drive and use machines. You may find that your vision is blurred for a time just after you put SAFLUTAN in your eye. Do not drive or use any tools or machines until your vision is clear.
45
Package leaflet: Information for the patient
SAFLUTAN® 15 micrograms/ml Eye drops, solution in single dose-container
Tafluprost
3. How to use SAFLUTAN
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor, pharmacist or nurse.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
See section 4.
What is in this leaflet:
1. What SAFLUTAN is and what it is used for
2. What you need to know before you use SAFLUTAN
3. How to use SAFLUTAN
4. Possible side effects
5. How to store SAFLUTAN
6. Contents of the pack and other information
1. What SAFLUTAN is and what it is used for
What kind of medicine is it and how does it work?
SAFLUTAN eye drops contain tafluprost, which belongs to a group of medicines called prostaglandin analogues. SAFLUTAN lowers the pressure in the eye. It is used when the pressure in the eye is too high.
What is your medicine for?
SAFLUTAN is used to treat a type of glaucoma called open angle glaucoma and also a condition known as ocular hypertension in adults. Both of these conditions are linked with an increase in the pressure within your eye and eventually they may affect your eyesight.
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is 1 drop of SAFLUTAN in the eye or eyes, once daily in the evening. Do not instil more drops or use more often than as instructed by your doctor. This may make SAFLUTAN less effective.
Only use SAFLUTAN in both eyes if your doctor told you to.
For use as eye drops only. Do not swallow.
Instructions for use:
When you start a new pouch:
Do not use the single-dose containers if the pouch is broken. Open the pouch along the dashed line. Write down the date you opened the pouch in the space reserved for the date on the pouch.
Every time you use SAFLUTAN:
1. Wash your hands.
2. Take the strip of containers from the pouch.
3. Detach one single-dose container from the strip.
4. Put the remaining strip back in the pouch and fold the edge to close the pouch.
5. Make sure that the solution is in the bottom part of the single-dose container.
6. To open the container, twist off the tab.
280
2. What you need to know before you use SAFLUTAN
Do not use SAFLUTAN:
if you are allergic to tafluprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using SAFLUTAN.
Please note that SAFLUTAN may have the following effects and that some of them may be permanent:
SAFLUTAN may increase the length, thickness, colour and/or number of your eyelashes and may cause unusual hair growth on your eyelids. SAFLUTAN may cause darkening of the colour of the skin around the eyes. Wipe off any excess solution from the skin. This will reduce the risk of skin darkening.
SAFLUTAN may change the colour of your iris (the coloured part of your eye). If SAFLUTAN is used in one eye only, the colour of the treated eye may permanently become different from the colour of the other eye.
Tell your doctor
if you have kidney problems if you have liver problems if you have asthma if you have other eye diseases.
Children and adolescents
SAFLUTAN is not recommended for children and adolescents below 18 years due to a lack of data on safety and efficacy.
Other medicines and SAFLUTAN
Tell your doctor or pharmacist if you are taking, have recently taken or might take other medicines.
If you use other medicines in the eye, leave at least 5 minutes between putting in SAFLUTAN and the other medication.
Pregnancy, breast-feeding and fertility
If you may become pregnant, you must use an effective method of birth control during SAFLUTAN therapy. Do not use SAFLUTAN if you are pregnant. You should not use SAFLUTAN if you are breast-feeding. Ask your doctor for advice.
7. Tilt your head backwards.
8. Place the tip of the container close to your eye.
9. Pull the lower eyelid downwards and look up.
10. Gently squeeze the container and let one drop fall into the space between the lower eyelid and the eye.
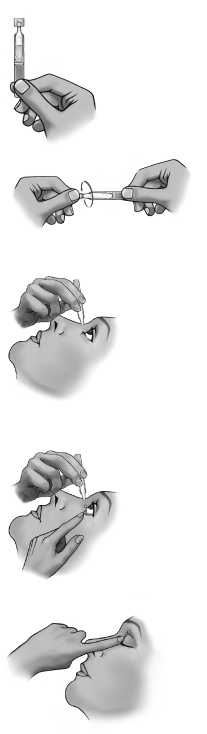
11. Close your eye for a moment and press the inner corner of the eye with your finger for about one minute.
This helps to prevent the eye drop from draining down the tear duct.
12. Wipe off any excess solution from the skin around the eye.
If a drop misses your eye, try again.
If your doctor has told you to use drops in both eyes, repeat steps 7 to 12 for your other eye.
The contents of one single-dose container are sufficient for both eyes. Discard the opened container with any remaining contents immediately after use.
If you use other medicines in the eye, leave at least 5 minutes between putting in SAFLUTAN and the other medication.
If you use more SAFLUTAN than you should, it is unlikely to cause you any serious harm. Put in your next dose at the usual time.

200

If the medicine is accidentally swallowed, please contact a doctor for advice.
Malta: ADR Reporting, The Medicines Authority, Post-Licensing Directorate, 203 Level 3, Rue D'Argens, GZR-1368 Gzira Website: www.medicinesauthority.gov.mt, e-mail: postlicensing. medicinesauthority@gov.mt
45
If you forget to use SAFLUTAN, use a single drop as soon as you remember, and then go back to your regular routine. Do not use a double dose to make up for a forgotten dose.
Do not stop using SAFLUTAN without asking your doctor. If you stop using SAFLUTAN, the pressure in the eye will increase again. This may cause a permanent injury to your eye.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
5. How to store SAFLUTAN
4. Possible side effects
280
Like all medicines, this medicine can cause side effects, although not ^-everybody gets them. Most side effects are not serious.
Common side effects
The following may affect up to 1 in 10 people:
Effects on the nervous system: headache.
Effects on the eye: itching of the eye irritation in the eye eye pain
redness of the eye
changes in the length, thickness and number of eyelashes dry eye
foreign body sensation in the eye discolouration of eyelashes redness of the eyelids
small spotlike areas of inflammation on the surface of the eye sensitivity to light watery eyes blurred vision
reduction in the eye's ability to see details change of colour of the iris (may be permanent).
Uncommon side effects
The following may affect up to 1 in 100 people:
Effects on the eye:
change of colour of the skin around the eyes puffy eyelids tired eyes
swelling of the eye's surface membranes eye discharge inflammation of the eyelids signs of inflammation inside the eye discomfort in the eye
pigmentation of the eye's surface membranes follicles in the surface membranes of the eye allergic inflammation abnormal sensation in the eye.
Effects on the skin and tissue under the skin: unusual hair growth on eyelids.
Not known: frequency cannot be estimated from the available data
Effects on the eye:
inflammation of the iris/uvea (middle layer of the eye) eyes appear sunken
Effects on the respiratory system:
worsening of asthma, shortness of breath
I n very rare cases, some patients with severe damage to the clear layer at the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium build-up during treatment.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom: Yellow Card Scheme at: www.mhra.gov.uk/yellowcard Ireland: HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2,
Tel: +353 1 6764971; Fax: +353 1 6762517, Website: www.hpra.ie; e-mail: medsafety@hpra.ie
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the single-dose container, pouch and the carton after 'Exp'. The expiry date refers to the last day of that month.
Store the unopened foil pouches in a refrigerator (2°C - 8°C). Do not open the pouch until you are about to start using the eye drops as unused containers in an open pouch must be discarded 28 days after first opening the pouch.
After opening the foil pouch:
• Keep the single-dose containers in the original foil pouch.
• Do not store above 25°C
• Discard unused single-dose containers after 28 days from date of first opening of the foil pouch
• Discard an opened single-dose container with any remaining solution immediately after use.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What SAFLUTAN contains
- The active substance is tafluprost. 1 ml of solution contains
15 micrograms of tafluprost. One single-dose container (0.3 ml) contains 4.5 micrograms of tafluprost. One drop (about 30 pl) contains about 0.45 micrograms of tafluprost.
- The other ingredients are glycerol, sodium dihydrogen phosphate dihydrate, disodium edetate, polysorbate 80 and water for injections. Hydrochloric acid and/or sodium hydroxide are added to adjust the pH.
What SAFLUTAN looks like and contents of the pack
SAFLUTAN is a clear, colourless liquid (solution) supplied in single-dose plastic containers, each containing 0.3 ml of solution. Ten single-dose containers are provided in one pouch. SAFLUTAN is supplied in packs containing 30 or 90 single-dose containers. Not all pack sizes may be marketed.
Marketing Authorisation Holder (UK/Ireland/Malta): Santen Oy, Niittyhaankatu 20, 33720 Tampere, Finland
Manufacturer: Laboratoire Unither, ZI La Guerie, 50211 Coutances Cedex, France
This medicinal product is authorised in the Member States of the EEA under the following names:
Bulgaria, Czech Republic, Denmark, Estonia, Finland, Hungary, Iceland, Latvia, Lithuania, Norway, Poland, Slovakia, Sweden Germany
Austria, Belgium, Cyprus, France, Greece, Ireland, Italy, Luxembourg, Malta, The Netherlands, Portugal, Romania, Slovenia, Spain, United Kingdom
This leaflet was last revised June 2015
0)
■c
0)
0)
o
tc
Taflotan
Taflotan sine Saflutan
