Saflutan 15 Micrograms/Ml Eye Drops Solution In Single-Dose Container
Out of date information, search anotherRead all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Your medicine is called Saflutan 15 micrograms/ml eye drops, solution in single-dose container but will be referred to as Saflutan throughout this leaflet.
What is in this leaflet:
^ What Saflutan is and what it is used for
What you need to know before you use Saflutan How to use Saflutan ^ Possible side effects ra How to store Saflutan ^6) Contents of the pack and other information
What Saflutan is and what it is used for
What kind of medicine is it and how does it work?
Saflutan eye drops contain tafluprost, which belongs to a group of medicines called prostaglandin analogues. Saflutan lowers the pressure in the eye.
It is used when the pressure in the eye is too high.
What is your medicine for?
Saflutan is used to treat a type of glaucoma called open angle glaucoma and also a condition known as ocular hypertension in adults. Both of these conditions are linked with an increase in the pressure within your eye and eventually they may affect your eyesight.
What you need to know before you use Saflutan
Do not use Saflutan:
• if you are allergic to tafluprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Saflutan.
Please note that Saflutan may have the following effects and that some of them may be permanent:
• Saflutan may increase the length, thickness, colour and/or number of your eyelashes and may cause unusual hair growth on your eyelids.
• Saflutan may cause darkening of the colour of the skin around the eyes. Wipe off any excess solution from the skin. This will reduce the risk of skin darkening.
• Saflutan may change the colour of your iris (the coloured part of your eye). If Saflutan is used in one eye only, the colour of the treated eye may permanently become different from the colour of the other eye.
Tell your doctor
• if you have kidney problems
• if you have liver problems
• if you have asthma
• if you have other eye diseases.
Children and adolescents
Saflutan is not recommended for children and adolescents below 18 years due to a lack of data on safety and efficacy.
Other medicines and Saflutan
Tell your doctor or pharmacist if you are taking, have recently taken or might take other medicines.
If you use other medicines in the eye, leave at least 5 minutes between putting in Saflutan and the other medication.
Pregnancy, breast-feeding and fertility
If you may become pregnant, you must use an effective method of birth control during Saflutan therapy. Do not use Saflutan if you are pregnant. You should not use Saflutan if you are breast-feeding. Ask your doctor for advice.
Driving and using machines
Saflutan has no influence on the ability to drive and use machines. You may find that your vision is blurred for a time just after you put Saflutan in your eye. Do not drive or use any tools or machines until your vision is clear.
How to use Saflutan
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is 1 drop of Saflutan in the eye or eyes, once daily in the evening. Do not instil more drops or use more often than as instructed by your doctor. This may make Saflutan less effective.
Only use Saflutan in both eyes if your doctor told you to.
For use as eye drops only. Do not swallow. Instructions for use:
When you start a new pouch:
Do not use the single-dose containers if the pouch is broken. Open the pouch along the dashed line.
Write down the date you opened the pouch in the space reserved for the date on the pouch.
Every time you use Saflutan:
1. Wash your hands.
2. Take the strip of containers from the pouch.
3. Detach one single-dose container from the strip.
4. Put the remaining strip back in the pouch and fold the edge to close the pouch.
5. Make sure that the solution is in the bottom part of the single-dose container.
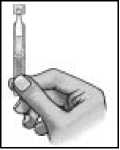
6.
To open the container, twist off the tab.
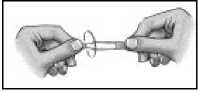
7. Tilt your head backwards.
8. Place the tip of the container close to your eye.
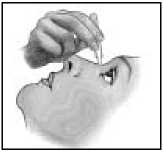
9. Pull the lower eyelid downwards and look up.
10. Gently squeeze the container and let one drop fall into the space between the lower eyelid and the eye.
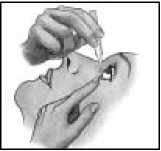
11. Close your eye for a moment and press the inner corner of the eye with your finger for about one minute.
This helps to prevent the eye drop from draining down the tear duct.
12. Wipe off any excess solution from the skin around the eye.
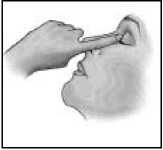
If your doctor has told you to use drops in both eyes, repeat steps 7 to 12 for your other eye.
The contents of one single-dose container are sufficient for both eyes.
Discard the opened container with any remaining contents immediately after use.
If you use other medicines in the eye, leave at least 5 minutes between putting in Saflutan and the other medication.
If you use more Saflutan than you should, it is
unlikely to cause you any serious harm. Put in your next dose at the usual time.
If the medicine is accidentally swallowed, please contact a doctor for advice.
If you forget to use Saflutan, use a single drop as soon as you remember, and then go back to your regular routine. Do not use a double dose to make up for a forgotten dose.
Do not stop using Saflutan without asking your doctor. If you stop using Saflutan, the pressure in the eye will increase again. This may cause a permanent injury to your eye.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
Q Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Most side effects are not serious.
Common side effects
The following may affect up to 1 in 10 people:
Effects on the nervous system:
• headache.
Effects on the eve:
• itching of the eye
• irritation in the eye
• eye pain
• redness of the eye
• changes in the length, thickness and number of eyelashes
• dry eye
• foreign body sensation in the eye
• discolouration of eyelashes
• redness of the eyelids
• small spot like areas of inflammation on the surface of the eye
• sensitivity to light
• watery eyes
• blurred vision
• reduction in the eye's ability to see details
• change of colour of the iris (may be permanent).
Uncommon side effects
The following may affect up to 1 in 100 people:
Effects on the eye:
• change of colour of the skin around the eyes
• puffy eyelids
• tired eyes
• swelling of the eye's surface membranes
• eye discharge
• inflammation of the eyelids
• signs of inflammation inside the eye
• discomfort in the eye
• pigmentation of the eye's surface membranes
• follicles in the surface membranes of the eye
• allergic inflammation
• abnormal sensation in the eye.
Effects on the skin and tissue under the skin:
• unusual hair growth on eyelids.
Not known: frequency cannot be estimated from the available data
Effects on the eye:
• inflammation of the iris/uvea (middle layer of the eye)
• eyes appear sunken
Effects on the respiratory system:
• worsening of asthma, shortness of breath
In very rare cases, some patients with severe damage to the clear layer at the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium build-up during treatment.
Q How to store Saflutan
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN
• Store unopened foil pouches between 2°C and 8°C (in a refrigerator).
Do not open the pouch until you are about to start using the eye drops as unused containers in an open pouch must be discarded 28 days after first opening the pouch.
• Do not use this medicine after the expiry date which is stated on the single-dose container, pouch and the carton label after EXP. The expiry date refers to the last day of that month.
After opening the foil pouch:
• Keep the single-dose containers in the original foil pouch.
• Do not store above 25°C
• Discard unused single-dose containers after 28 days from date of first opening of the foil pouch.
• Discard an opened single-dose container with any remaining solution immediately after use.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
What Saflutan contains
The active substance is tafluprost.
Each ml of solution contains 15 micrograms of tafluprost.
One single-dose container (0.3ml) of eye drops, solution, contains 4.5 micrograms of tafluprost.
The other ingredients are glycerol, sodium dihydrogen phosphate dihydrate, disodium edetate, polysorbate 80, sodium hydroxide and/or hydrochloric acid to adjust pH and water for injections.
What Saflutan looks like and contents of the pack
Saflutan is a clear, colourless solution supplied in single-dose plastic containers, each containing 0.3 ml of solution.
Each pack contains 30 single-dose containers.
Manufacturer and Licence Holder
Manufactured by Merck Sharpe & Dohme B.V., Waarderweg 39, 2031 BN Haarlem,
The Netherlands and is procured from within the EU and repackaged by the Product Licence Holder: Lexon (UK) Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 0RE
Saflutan is a registered trademark of Merck Sharp & Dohme Corp
PL 15184/1524
Leaflet revision date: 27/05/15
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Ref: 1524/270515/1/B