Sodium Chloride 0.9%W/V Solution For Intravesical Use
14-225 Sodium Chloride leaflet V2_Layout 1 28/11/2014 11:31 Page 1
Artwork Information
|
Product Title: |
Sodium Chloride leaflet | ||
|
Date: |
28-11-14 |
Product Size: |
420 x297mm |
|
Label Number: |
14-225 Version 2 |
Colours Used: |
CMYK |
|
Fonts Used: |
Bosis | ||
|
Font size: |
10pt | ||
Magenta text, keylines and shading are NOT to be printed

PACKAGE LEAFLET: INFORMATION FOR THE USER
□ ALLIANCE
Sodium Chloride 0.9% w/v Solution for Intravesical Use
(Sodium Chloride)
Read all of this leaflet carefully because it contains important information for you.
This medicine is available without prescription. However, you still need to use Sodium Chloride Solution carefully to get the best results from it. Keep this leaflet. You may need to read it again. Ask your doctor, nurse or pharmacist if you need more information or advice. You must contact a doctor if your symptoms worsen or do not improve. If any of the side effects become serious, or if you notice any side effect not listed in this leaflet, please tell your doctor, nurse or pharmacist.
In this leaflet:
1. What Sodium Chloride Solution is and what it is used for
2. Before you use Sodium Chloride Solution
3. How to use Sodium Chloride Solution
4. Possible side effects
5. How to store Sodium Chloride Solution
6. Further information
1. WHAT SODIUM CHLORIDE SOLUTION IS AND WHAT IT IS USED FOR
Your medicine is called Sodium Chloride Solution.
It belongs to a group of salt containing drugs that can be placed into the urinary bladder.
Sodium Chloride Solution is used to reconstitute another drug for administration into the urinary bladder through a catheter.
2. BEFORE YOU USE SODIUM CHLORIDE SOLUTION
Before you start using Sodium Chloride Solution read the information which is given below. If you think that any of this information applies to you, or are not sure, tell your doctor, nurse or pharmacist:
Do not use Sodium Chloride Solution if: you are allergic (hypersensitive) to sodium chloride or any of the other ingredients of Sodium Chloride Solution. Take special care with Sodium Chloride Solution Before treatment with Sodium Chloride Solution extra care will be taken if your bladder or urethra are fragile, for example in cases of advanced bladder cancer.
Using other medicines
There are no other medicines known that cause problems when they are taken when you are also using Sodium Chloride Solution.
Tell your doctor, nurse or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Using Sodium Chloride Solution with food and drink You can eat and drink as normal when using Sodium Chloride Solution.
Pregnancy and breast-feeding
If you are or could be pregnant, tell your doctor before
treatment starts.
Ask your doctor, nurse or pharmacist for advice before taking any medicine.
Driving and using machines
Your ability to drive a car or operate machinery will not be affected following administration of Sodium Chloride Solution.
3. HOW TO USE SODIUM CHLORIDE SOLUTION
Sodium Chloride Solution will be given to you by a doctor or nurse. Each 50ml bag is for single use only and any unused portion will be discarded.
Your doctor will have decided how much and for how long you need to be given Sodium Chloride Solution.
If you have any further questions on the use of this product, ask your doctor, nurse or pharmacist.
4. POSSIBLE SIDE EFFECTS
There are no known side effects caused by Sodium Chloride Solution.
Reporting of side effects
If you get any side effects, talk to your doctor, nurse or pharmacist.
This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the internet atwww.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE SODIUM CHLORIDE SOLUTION
• Keep out of the reach and sight of children.
• Do not use Sodium Chloride Solution after the expiry date which is stated on the bag. The expiry date refers to the last day of that month.
• Do not store above 25°C and do not freeze.
• Store in the original container.
• Do not use Sodium Chloride Solution if you notice that the solution contains any particles or has become cloudy.
• Do not use if the container has become damaged.
Summary of Product Characteristics
ALLIANCE
PRODUCT SUMMARY
1. TRADE NAME OF THE MEDICINAL PRODUCT
Sodium Chloride 0.9% w/v Solution for Intravesical Use.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Sodium Chloride 0.9% w/v
Each 50ml bag contains 0.45 g of sodium chloride.
For a full list of excipients, see 6.1.
3. PHARMACEUTICAL FORM
Solution for intravesical use.
Clear colourless liquid.
Physico-chemical properties:
pH : 3.5 - 5.5
Osmolarity : 15.4 m0sm/50ml Na+ and CD : 7.7 mmol/50ml
4. CLINICAL PARTICULARS
4.1. THERAPEUTIC INDICATIONS
As a diluent for the administration of drugs within the bladder.
4.2. POSOLOGY AND METHOD OF ADMINISTRATION
Adults, children and the elderly:
The volume to be used, frequency and duration of the treatment depend on the nature of the drug administered and the clinical condition of the patient. Route of Administration:
Urethral.
Refer to section 6.6, 'Special precautions for disposal and other handling' for further information.
4.3. CONTRAINDICATIONS
Hypersensitivity to any of the excipients.
4.4.SPECIAL WARNINGS AND PRECAUTIONS FOR USE.
Caution should be exercised in cases of advanced carcinoma of the bladder, as the bladder may be fragile. An intact urethra is required.
Ensure proper mixing of any added medication.
Caution should be exercised in the attachment and clamping of both the active medication and the urinary catheter.
4.5.INTERACTIONS WITH OTHER MEDICAMENTS AND OTHER FORMS OF INTERACTION
None.
4.6. PREGNANCY AND LACTATION
No known effect.
Caution should be exercised when prescribing to pregnant women.
4.7. EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
None known.
4.8.UNDESIRABLE EFFECTS
None known.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme. Tel: Freephone 0808 100 3352.
Website: www.mhra.gov.uk/yellowcard.
4.9.OVERDOSE
Not applicable.
5. PHARMACOLOGICAL PROPERTIES
5.1. PHARMACODYNAMIC PROPERTIES
ATC Code : B05C B 01
Irrigating solutions, salt solutions
There are no pharmacodynamic properties of relevance
for this product.
5.2. PHARMACOKINETIC PROPERTIES
There are no pharmacokinetic properties applicable for this product.
5.3. PRECLINICAL SAFETY DATA
There are no preclinical data of relevance to the prescriber, which are additional to that already included elsewhere in the SPC.
6. PHARMACEUTICAL PARTICULARS 6.1.LIST OF EXCIPIENTS
Water for Injections 6.2.INCOMPATIBILITIES None known.
6.3.SHELF LIFE
24 months.
6.4.SPECIAL PRECAUTIONS FOR STORAGE
Do not store above 25°C. Do not freeze. Store in original container.
6.5.NATURE AND CONTENTS OF CONTAINER
A 50ml infusion bag constructed from 2 PVC tubes welded at each end. At one end of the bag, 2 PVC tubes are welded in place. At the other end of the bag there is one, centrally placed suspension hole in the weld web.
A breakable male luer is located at the end of one of the tubes and a frangible section is located at the other end. A universal vial connector connects on to a male luer. It has a threaded stopper end and a covered needle which can be used to transfer fluid from a vial. A blue connector is fitted into the administration tube of the bladder irrigation bag.
6.6.SPECIAL PRECAUTIONS FOR DISPOSAL AND OTHER HANDLING
Do not use unless the solution is clear and the container is intact.
Each 50ml bag is for single use only and any unused portion should be discarded.
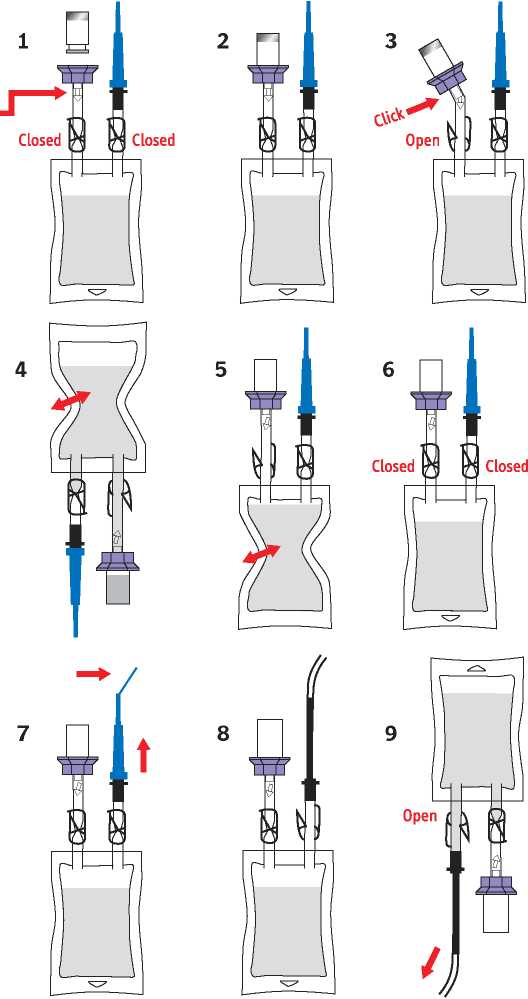
INSTRUCTIONS FOR USE
1 Take the bag with the Sodium Chloride 0.9% w/v solution out of the packaging. Check that the bag is at room temperature and that there are no particles in the bag. Ensure that both clamps are closed. Remove the flip-top cap from the drug vial.
Check that the Luer lock at this junction is secure.
2 Push the spike of the lilac coloured connector into the centre of the stopper of the drug vial until all six grips click into place around the neck of the vial. Check once again that the Luer lock is secure.
3 Break the cone below the lilac connector and open the clamp.
4 Invert the bag with the vial attached and gently squeeze the bag several times. The sodium chloride solution will flow into the vial. Gently agitate the vial to dissolve the contents.
5 Turn the bag and vial upwards again and gently press the air out of the bag into the drug vial. As the pressure subsides, the dissolved drug solution will flow into the bag. Repeat this step once or twice until the vial contents are removed.
6 Close the clamp between the vial and the bag and check that the clamp below the blue capped catheter adapter is also closed.
7 Bend the end of the blue cap to break the tip of the catheter adapter and remove the blue cap.
8 Push the connecting piece of the catheter adapter into the catheter. Release the clamp below the catheter adapter.
9 Instill the solution slowly into the bladder. Any equipment or material which has come into contact with the solution must be disposed of in accordance with local policy.
ADMINISTRATIVE DATA
7. MARKETING AUTHORISATION HOLDER
Alliance Pharmaceuticals Limited, Avonbridge House Bath Road, Chippenham, Wiltshire, SN15 2BB, UK.
8. MARKETING AUTHORISATION NUMBER
PL 16853/0123
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
6th January 2011
10. DATE OF REVISION OF THE TEXT
November 2014
6. FURTHER INFORMATION
What Sodium Chloride Solution contains
The active substance is sodium chloride. Each bag contains 0.45 grams of sodium chloride. The other ingredient is water for injections.
What Sodium Chloride Solution looks like and contents of the pack
Sodium Chloride Solution is a clear, sterile solution that is contained in a PVC bag. The bag contains 50ml of solution.
Marketing Authorisation Holder and Manufacturer
The marketing authorisation holder for Sodium Chloride Solution is:
Alliance Pharmaceuticals Limited, Avonbridge House, Bath Road, Chippenham, Wiltshire, SN15 2BB, UK.
The company responsible for manufacturing this product is: Terumo BCT Ltd, Larne, Co. Antrim, BT40 2SH United Kingdom.
This leaflet was last revised in November 2014.