Sodium Chloride 0.9%W/V Solution For Intravesical Use
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Sodium Chloride 0.9% w/v Solution for Intravesical Use.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Sodium Chloride 0.9%w/v
Each 50 ml bag contains 0.45 g of sodium chloride.
For a full list of excipients, see 6.1.
3 PHARMACEUTICAL FORM
Solution for intravesical use.
Clear colourless liquid.
Physico-chemical properties: pH: 3.5 - 5.5
Osmolarity: 15.4 mOsm/50 ml
Na+ and Cl- : 7.7 mmol/50 ml
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
As a diluent for the administration of drugs within the bladder.
4.2 Posology and method of administration
Adults, children and the elderly:
The volume to be used, frequency and duration of the treatment depend on the nature of the drug administered and the clinical condition of the patient.
Route of Administration: Urethral.
Refer to section 6.6, Special precautions for disposal and other handling’ for further information.
4.3 Contraindications
Hypersensitivity to any of the excipients.
4.4 Special warnings and precautions for use
Caution should be exercised in cases of advanced carcinoma of the bladder, as the bladder may be fragile.
An intact urethra is required.
Ensure proper mixing of any added medication.
Caution should be exercised in the attachment and clamping of both the active medication and the urinary catheter.
4.5 Interaction with other medicinal products and other forms of interaction
None.
4.6 Pregnancy and lactation
No known effect.
Caution should be exercised when prescribing to pregnant women.
4.7 Effects on ability to drive and use machines
None known.
4.8 Undesirable effects
None known.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme. Tel: Freephone 0808 100 3352. Website: www.mhra.gov .uk/yellowcard.
4.9 Overdose
Not applicable.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
There are no pharmacodynamic properties of relevance for this product. ATC Code: B05C B 01 Irrigating solutions, salt solutions
5.2 Pharmacokinetic properties
There are no pharmacokinetic properties applicable for this product.
Preclinical safety data
5.3
There are no preclinical data of relevance to the prescribe^ which are additional to that already included elsewhere in the SPC.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Water for Injections
6.2 Incompatibilities
None known.
6.3 Shelf life
24 months.
6.4 Special precautions for storage
Do not store above 25°C. Do not freeze.
Store in original container.
6.5 Nature and contents of container
A 50ml infusion bag constructed from 2 PVC tubes welded at each end. At one end of the bag, 2 PVC tubes are welded in place. At the other end of the bag there is one, centrally placed suspension hole in the weld web. A breakable male luer is located at the end of one of the tubes and a frangible section is located at the other end. A universal vial connector connects on to a male luer. It has a threaded stopper end and a covered needle which can be used to transfer fluid from a vial. A blue connector is fitted into the administration tube of the bladder irrigation bag.
6.6 Special precautions for disposal
Do not use unless the solution is clear and the container is intact.
Each 50ml bag is for single use only and any unused portion should be discarded.
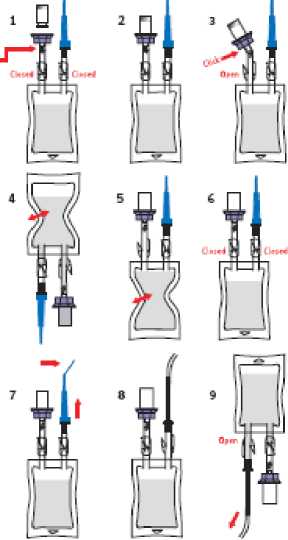
INSTRUCTIONS FOR USE
1 Tata iIk tug widi Hu Sodium CNnrtde- &9% w/v solution cut etI rha packaging. Chock, tiiac eha teg b ai :umparaura and due ehas- arg m panicles In rha bug Ensure lhaib-Jdi damps arg dnsod. Ramwo ihg Hip-cop cap tTom iha drugvUL Chock that dig LuarLocfc at dlls junction Is sacura.
1 Push dv 5pots of ctd ILac oannacuc tnin rhs-
csiwi- w tra steppgr it dig dnigvial unci all sh grips dJcfc Inn- placa amend rta ruck o! iha vial Shade ones again due dig Luar lock Is sacura.
3 Break die- oers- is lew dig Ulas oonnacmr and apan rha damp.
4 Inw-re chg lag with eha vtal acuds-d and gaudy squeaia die- bag savaral dmas. Its- sedium cNLartda solution will Hew Imo dig vlaL Sanely agfcara chg vial m dissedva els concetm.
5 Tun dig hag and al upwards again and partly press ihe air out of die- bag kin its- drug vial As dig pressure subsrdc^ iha dssobad drug sriudon wIJ. Mow miocha bug Kapa-at ties sup meg or twica ixiUI ds-vlai.
cdmenu are removal
6 Closa rha darnpbecwwn rJsvIaiand rha bag and die-do ihai die- clarrp Pajcw its- blua capped cachsuf adapier Is also closed.
7 Bend rfe endoi ihc bkrecap to tarsal-; Us dp of die □ctaur adaprarand remove-cha blue cap.
& Push dw comecnng pogcg o! thg cadiaref adapter Inin die- carhetat Retaasa- die- clarrp bafcw rhe- carhatar adapts*
9 Ir&cUdia so lira on slowly imn rha tladdot Any
e-gLipmarit or macadal winch tes oanrs into contact wnh die- solution muse be- disposed crl ki accorduioQ<wtdi local policy.
7 MARKETING AUTHORISATION HOLDER
Alliance Pharmaceuticals Limited
Avonbridge House
Bath Road
Chippenham
Wiltshire,
SN15 2BB UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 16853/0123
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION
07 March 2011
10 DATE OF REVISION OF THE TEXT
05/01/2015