Spiriva Respimat 2.5 Microgram Inhalation Solution
Daily use of your Spiriva Respimat inhaler What if...
You will need to use this inhaler only ONCE A DAY. Each time you use it take TWO PUFFS.

I Hold the Spiriva Respimat inhaler upright, with the green cap (A) closed, to avoid accidental release of dose. Turn the base (G) in the direction of the black arrows on the label until it clicks (half a turn).
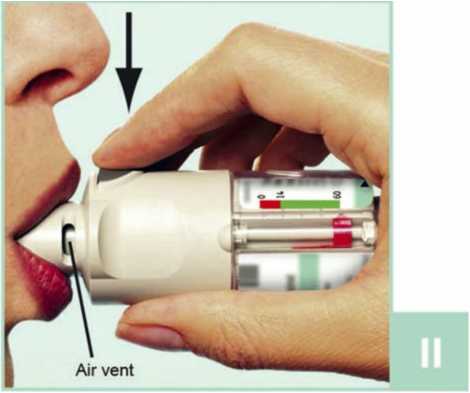
II Open the green cap (A) until it snaps fully open. Breathe out slowly and fully, and then close your lips around the end of the mouthpiece without covering the air vents (C). Point your Spiriva Respimat inhaler to the back of your throat.
While taking in a slow, deep breath through your mouth, press the dose release button (D) and continue to breathe in slowly for as long as you can. Hold your breath for 10 seconds or for as long as comfortable.
III Repeat steps I and II so that you get the full dose.
You will need to use this inhaler only ONCE A DAY.
Close the green cap until you use your Spiriva Respimat inhaler again.
If Spiriva Respimat inhaler has not been used for more than 7 days release one puff towards the ground. If Spiriva Respimat inhaler has not been used for more than 21 days repeat steps 4 to 6 until a cloud is visible. Then repeat steps 4 to 6 three more times.
When to get a new Spiriva Respimat inhaler
|
What if... |
Reason |
What to do |
|
I can't turn the base easily. |
a) The Spiriva Respimat inhaler is already prepared and ready to use. b) The Spiriva Respimat inhaler is locked after 60 puffs (30 medicinal doses). |
a) The Spiriva Respimat inhaler can be used as it is. b) Prepare and use your new Spiriva Respimat inhaler. |
|
The cap is fully pulled off and apart from the inhaler. |
While opening the cap it was pulled too hard. |
The cap can easily be attached again. |
|
I can’t press the dose release button. |
The clear base has not been turned. |
Turn the clear base until it clicks. (half a turn) |
|
The clear base springs back after I have turned it. |
The clear base was not turned far enough. |
Prepare the Spiriva Respimat inhaler for use by turning the clear base until it clicks, (half a turn) |
|
I can turn the clear base past the point where it clicks. |
Either the dose release button has been pressed, or the clear base has been turned too far. |
With the green cap closed, turn the base until it clicks, (half a turn) |
Clean the mouthpiece including the metal part inside the mouthpiece with a damp cloth or tissue only, at least once a week. Any minor discolouration in the mouthpiece does not affect the performance of your Spiriva Respimat inhaler.
If necessary, wipe the outside of your Spiriva Respimat inhaler with a damp cloth.
The Spiriva Respimat Inhaler must not be disassembled after inserting the cartridge and replacing the clear base.
Do not touch the piercing element inside the base.
Boehringer Ingelheim Pharma GmbH & Co. KG D-55216 Ingelheim Germany.

The Spiriva Respimat inhaler contains 60 puffs (30 medicinal doses). The dose indicator shows approximately how much medication is left. When the pointer enters the red area of the scale, there is, approximately, medication for 7 days left (14 puffs). This is when you need to get a new Spiriva Respimat inhaler prescription.
Once the dose indicator has reached the end of the red scale (i.e. all 3 doses have been used), the Spiriva Respimat inhaler locks automatically - no more doses can be released. At this point, the base cannot be turned any further.
At the latest, three months after use the Spiriva Respimat inhaler should be discarded even if not all medication has been used
Spiriva® Respimat®2.5 microgram, inhalation solution
(tiotropium bromide)
Patient Information Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Your medicine is called Spiriva Respimat 2.5 microgram inhalation solution and will be referred to as Spiriva Respimat throughout the rest of this leaflet.
Q What Spiriva Respimat is and what it is used for Q What you need to know before you take Spiriva Respimat Q How to take Spiriva Respimat Q Possible side effects Q How to store Spiriva Respimat Q Contents of the pack and other information
Q What Spiriva Respimat is and what it is used for
Spiriva Respimat helps people who have chronic obstructive pulmonary disease (COPD) or asthma to breathe more easily. COPD is a long-term lung disease that causes shortness of breath and coughing. The term COPD is associated with the conditions chronic bronchitis and emphysema. Asthma is a long-term disease characterised by airway inflammation and narrowing of the airways. As COPD and asthma are long-term diseases you should take Spiriva Respimat every day and not only when you have breathing problems or other symptoms. When used to treat asthma you should use Spiriva Respimat in addition to so-called inhaled corticosteroids and long-acting IJ2 agonists.
Spiriva Respimat is a long-acting bronchodilator that helps to open your airways and makes it easier to get air in and out of the lungs. Regular use of Spiriva Respimat can also help you when you have on-going shortness of breath related to your disease, and will help to minimise the effects of the disease on your everyday life. Daily use of Spiriva Respimat will also help to prevent any sudden, short-term worsening of your COPD symptoms which may last for several days.
For correct dosing of Spiriva Respimat please see section 3. How to take Spiriva Respimat and the instructions for use provided on the other side of the leaflet.
Q What you need to know before you take Spiriva Respimat Please read the following questions carefully.
If you can answer any of these questions with ‘Yes’ please discuss this with your doctor before taking Spiriva Respimat
• are you allergic (hypersensitive) to tiotropium, atropine or similar drugs such as ipratropium or oxitropium?
• are you taking any other medicinal products containing ipratropium or oxitropium?
• are you pregnant, do you think you are pregnant, or are you breast-feeding?
• are you suffering from blurred vision, eye pain and/or red eyes, prostate problems or have difficulty passing urine?
• do you have any kidney problems?
• have you suffered from a myocardial infarction during the last 6 months or from any unstable or life threatening irregular heart beat or severe heart failure within the past year?
• if you are allergic (hypersensitive) to tiotropium, its active ingredient or any of the other ingredients of this medicine (listed in section 6)
• if you are allergic (hypersensitive) to atropine or substances related to it, e.g. ipratropium or oxitropium
Talk to your doctor before taking Spiriva Respimat.
When taking Spiriva Respimat take care not to let any spray enter your eyes. This may result in eye pain or discomfort, blurred vision, seeing halos around lights or coloured images in association with red eyes (i.e. narrow angle glaucoma). Eye symptoms may be accompanied by headache, nausea or vomiting. Wash your eyes in warm water, stop using tiotropium bromide and immediately consult your doctor for further advice.
If your breathing has got worse or if you experience rash, swelling or itching directly after using your inhaler, stop using it and tell your doctor immediately.
Dry mouth which has been observed with anti-cholinergic treatment may in the long term be associated with dental caries. Therefore, please remember to pay attention to oral hygiene.
Spiriva Respimat is indicated for the maintenance treatment of your chronic obstructive pulmonary disease or asthma. Do not use this medicine to treat a sudden attack of breathlessness or wheezing. Your doctor should have given you another inhaler (“rescue medication”) for this. Please follow the instructions your doctor has given you.
If you have been prescribed Spiriva Respimat for your asthma it should be added on to inhaled corticosteroids and long-acting IJ2 agonists. Continue taking the inhaled corticosteroids as prescribed by your doctor, even if you feel better.
In case you have suffered from a myocardial infarction during the last 6 months or from any unstable or life threatening irregular heart beat or severe heart failure within the past year, please, inform your doctor. This is important to decide if Spiriva is the right medicine for you to take.
Do not take Spiriva Respimat more frequently than once daily.
You should also contact your doctor if you feel that your breathing is worsening.
If you have cystic fibrosis, tell your doctor because Spiriva Respimat could make your cystic fibrosis symptoms worse.
Spiriva Respimat is not recommended for children and adolescents under 18 years.
Other medicines and Spiriva Respimat
Please tell your doctor or pharmacist if you are taking, or have recently taken, any other medicines, including medicines obtained without a prescription.
In particular, please tell your doctor or pharmacist if you are taking/have taken anticholinergic drugs, e.g. ipratropium or oxitropium.
No interaction side effects have been reported when Spiriva Respimat has been taken with other products used to treat COPD such as reliever inhalers (e.g. salbutamol), methylxanthines (e.g. theophylline), antihistamines, mucolytics (e.g. ambroxol), leukotriene modifiers (e.g. montelukast), cromones, anti-lgE treatment (e.g. omalizumab) and/or inhaled or oral steroids (e.g. budesonide, prednisolone).
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. You should not use this medicine unless specifically recommended by your doctor.
No studies on the effects and the ability to drive and use machines have been performed. In case dizziness or blurred vision occurs the ability to drive and use machinery may be influenced.
Q How to take Spiriva Respimat
Always take this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Spiriva Respimat is for inhalation use only.
The recommended dose for adults is:
Spiriva Respimat is effective for 24 hours so you will need to use Spiriva Respimat only ONCE A DAY, if possible at the same time of the day. Each time you use it take TWO PUFFS.
As COPD and asthma are long-term diseases take Spiriva Respimat every day and not only when you experience breathing problems. Do not take more than the recommended dose.
Spiriva Respimat is not recommended for use in children and adolescents below 18 years due to lack of data on safety and efficacy.
Make sure that you know how to use your Spiriva Respimat inhaler properly. The instructions for use of the Spiriva Respimat inhaler are provided on the other side of this leaflet.
If you take more Spiriva Respimat than you should
If you take more Spiriva Respimat than two puffs in one day talk to your doctor immediately. You may be at a higher risk of experiencing a side effect such as dry mouth, constipation, difficulties passing urine, increased heart beat or blurred vision.
If you forget to take Spiriva Respimat
If you forget to take your daily dose (TWO PUFFS ONCE A DAY), don’t worry. Take it as soon as you remember but do not take two doses at the same time or on the same day. Then take your next dose as usual.
If you stop taking Spiriva Respimat
Before you stop taking Spiriva Respimat, you should talk to your doctor or your pharmacist. If you stop taking Spiriva Respimat the signs and symptoms of COPD may worsen.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
POM
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Evaluation of the side effects is based on the following frequencies:
Common: may affect up to 1 in 10 people Uncommon: may affect up to 1 in 100 people Rare: may affect up to 1 in 1,000 people
Not known: frequency cannot be estimated from the available data
The side effects described below have been experienced by people taking this medicine and they are listed according to frequency as either common, uncommon, rare or not known
|
Side effect |
Frequency COPD |
Frequency Asthma |
|
Dry mouth: this is usually mild |
Common |
Common |
|
Dizziness |
Uncommon |
Uncommon |
|
Headache |
Uncommon |
Uncommon |
|
Difficulty in sleeping (insomnia) |
Rare |
Uncommon |
|
Irregular heart beat (atrial fibrillation, supraventricular tachycardia) |
Rare |
Not known |
|
Feeling your heartbeat (palpitations) |
Rare |
Uncommon |
|
Faster heart beat (tachycardia) |
Rare |
Not known |
|
Cough |
Uncommon |
Uncommon |
|
Nosebleed (epistaxis) |
Rare |
Not Known |
|
Inflammation of the throat (pharyngitis) |
Uncommon |
Uncommon |
|
Hoarseness (dysphonia) |
Uncommon |
Uncommon |
|
Tightness of the chest, associated with coughing, wheezing or breathlessness immediately after inhalation (bronchospasm) |
Rare |
Uncommon |
|
Constipation |
Uncommon |
Rare |
|
Fungal infections of the oral cavity and throat (oropharyngeal candidiasis) |
Uncommon |
Uncommon |
|
Difficulties swallowing (dysphagia) |
Rare |
Not known |
|
Rash |
Uncommon |
Rare |
|
Itching (pruritus) |
Uncommon |
Rare |
|
Difficulties passing urine (urinary retention) |
Uncommon |
Not known |
|
Painful urination (dysuria) |
Uncommon |
Not known |
|
Seeing halos around lights or coloured images in association with red eyes (glaucoma) |
Rare |
Not known |
|
Increase of the measured eye pressure |
Rare |
Not known |
|
Blurred vision |
Rare |
Not know |
|
Inflammation of the larynx (laryngitis) |
Rare |
Not known |
|
Heart burn (gastrooesophageal reflux disease) |
Rare |
Not Known |
|
Dental caries |
Rare |
Not known |
|
Inflammation of the gums (gingivitis) |
Rare |
Rare |
|
Inflammation of the tongue (glossitis) |
Rare |
Not known |
|
Inflammation of the mouth (stomatitis) |
Not known |
Rare |
|
Serious allergic reaction which causes swelling of the mouth and face or throat (angioneurotic oedema) |
Rare |
Rare |
|
Nettle rash (urticaria) |
Rare |
Rare |
|
Infections or ulcerations of the skin |
Rare |
Not known |
|
Dryness of the skin |
Rare |
Not known |
|
Hypersensitivity, including immediate reactions |
Not known |
Rare |
|
Infections of the urinary tract |
Rare |
Not known |
|
Depletion of body water (dehydration) |
Not known |
Not known |
|
Inflammation in sinuses (sinusitis) |
Not known |
Not known |
|
Blockage of intestines or absence of bowel movements (intestinal obstruction, including ileus paralytic) |
Not known |
Not known |
|
Feeling sick (nausea) |
Not known |
Not known |
|
Severe allergic reaction (anaphylactic reaction) |
Not known |
Not known |
|
Swelling of joint |
Not known |
Not known |
Immediate allergic reactions such as rash, nettle rash (urticaria), swelling of the mouth and face or sudden difficulties in breathing (angioneurotic oedema) or other hypersensitivity reactions (such as sudden reduction of your blood pressure or dizziness) may occur individually or as part of severe allergic reaction (anaphylactic reaction) after administration of Spiriva Respimat. If any of these occur, please consult your doctor immediately
In addition, in common with all inhaled medicines, some patients may experience an unexpected tightness of the chest, coughing, wheezing or breathlessness immediately after inhalation (bronchospasm).
If you get any side effects, talk to your doctor or pharmacist.This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (Tel: Freephone 0808 100 3352 or Website: www.mhra.aov.uk/vellowcard1. By reporting side effects you can help provide more information on the safety of this medicine.
O How to store Spiriva Respimat Keep out of the sight and reach of children.
Do not use Spiriva Respimat after the expiry date which is stated on the carton and on the inhaler label. The expiry date refers to the last day of the month. Spiriva Respimat inhaler should be discarded at the latest 3 months after first use (see Instructions for Use overleaf).
Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
If your medicine becomes discoloured or show any other signs of deterioration, ask your pharmacist who will advise you what to do.
Q Contents of the pack and other information What Spiriva Respimat contains:
The active substance is tiotropium. The delivered dose is 2.5 micrograms tiotropium (as bromide monohydrate) per puff (2 puffs comprise one medicinal dose).
The other ingredients are: Benzalkonium chloride, disodium edetate, purified water, and hydrochloric acid 3.6% for pH adjustment.
What Spiriva Respimat looks like and contents of the pack
The Cardboard carton contains one inhaler and one aluminium cylindrical cartridge containing a clear colourless solution for inhalation. A plastic cap is present on both ends of the inhaler: a green cap covering the mouth piece; and a transparent cap where the cartridge is to be inserted. The inhaler has an indicator on the side showing the number of doses left.
Single pack: 1 Respimat inhaler and 1 cartridge, providing 60 puffs (30 medicinal doses)
Manufacturer and Licence Holder
This medicine is manufactured by Boehringer Ingelheim Pharma GmbH &
Co. KG, Binger Strasse 173, D-55216 Ingelheim am Rhein, Germany and is procured from within the EU. Product Licence Holder: Lexon UK Limited, Unit 18 Oxleasow Road, East Moons Moat, Redditch, Worcestershire,
B98 ORE.
If you have any questions or are not sure about anything, ask your doctor or pharmacist. They will have additional information about this medicine and will be able to advise you.
Spiriva® Respimat® is a registered trademark of Boehringer ingelheim Pharma GmbH & Co. KG.
PL 15184/1645 - Spiriva Respimat 2.5 microgram, inhalation solution
Leaflet revision date: 08/06/16
Blind or partially sighted? Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Instructions for Use
Spiriva Respimat Inhaler
How to use your Spiriva Respimat Inhaler
This leaflet explains how to use and care for your Spiriva Respimat inhaler. Please read and carefully follow these instructions. See also section 3. How to take Spiriva Respimat on the other side of this leaflet.
The Spiriva Respimat inhaler releases medication slowly and gently, making it easy to inhale it into your lungs.
The Spiriva Respimat inhaler enables you to inhale the medicine contained in a cartridge. The full cartridge provides 60 puffs (30 medicinal doses). You will need to use this inhaler only ONCE A DAY, if possible at the same time of the day. Each time you use it take TWO PUFFS.
There is enough medicine for 30 days when it is used according to the directions for use. In the box you will find the Spiriva Respimat inhaler and the Spiriva Respimat cartridge. Before the Spiriva Respimat inhaler is used for the first time, the cartridge provided must be inserted.
Ca p (A)
Mouthpiece (B) Air vent (C)
Dose release button (D)
Safety catch (E)
Clear base (G)
Piercing element (I)
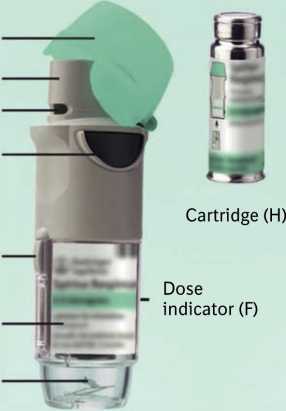
Spiriva Respimat inhaler and the Spiriva Respimat cartridge
1) Inserting the cartridge
The following steps 1-6 are necessary before first use:
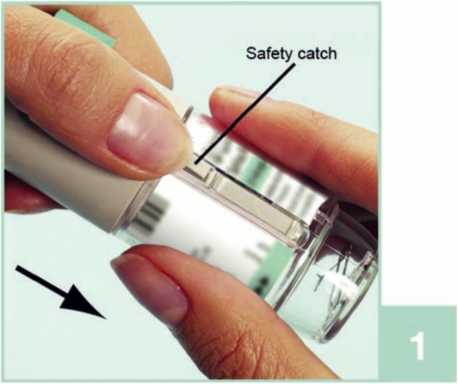
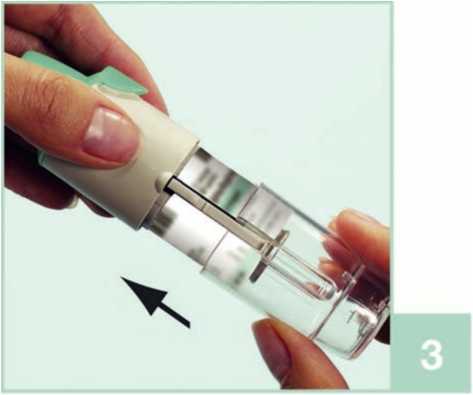
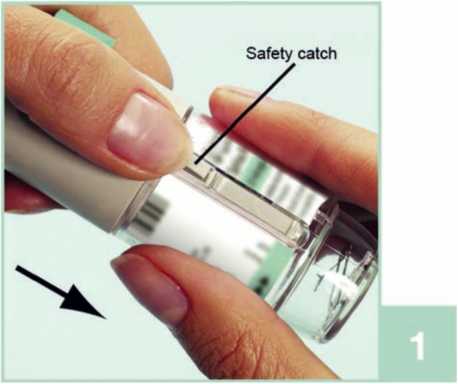
1 With the green cap (A) closed, press the safety catch (E) while pulling off the clear base (G).
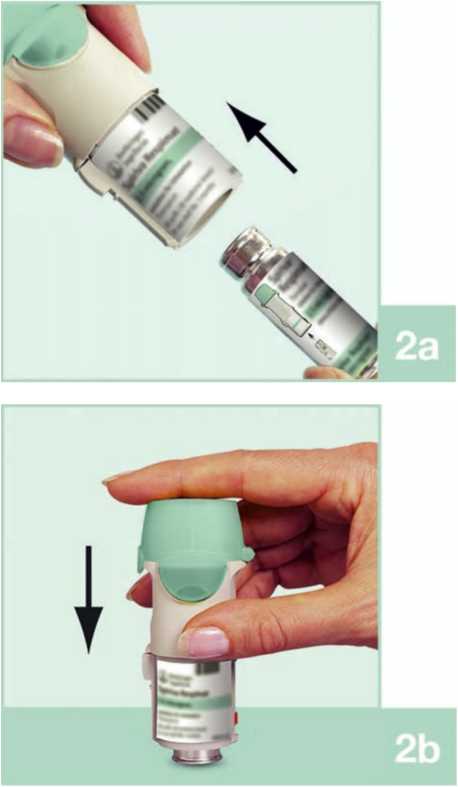
2. Take the cartridge (H) out of the box. Push the narrow end of the cartridge into the inhaler until it clicks into place. The cartridge should be pushed firmly against a firm surface to ensure that it has gone all the way in (2b).
The cartridge will not be flush with the inhaler, you will still see the silver ring of the lower end of the cartridge.
Do not remove the cartridge once it has been inserted into the inhaler
3. Replace the clear base (G). Do not remove the clear base again.
2) To prepare the Spiriva Respimat inhaler for first-time use

4. Hold the Spiriva Respimat inhaler upright, with the green cap (A) closed. Turn the base (G) in the direction of the red arrows on the label until it clicks (half a turn).

5. Open the green cap (A) until it snaps fully open.

6. Point the Spiriva Respimat inhaler towards the ground. Press the dose release button (D). Close the green cap (A).
Repeat steps 4, 5 and 6 until a cloud is visible.
Then repeat steps 4, 5 and 6 three more times to ensure the inhaler is prepared for use.
Your Spiriva Respimat inhaler is now ready to use.
These steps will not affect the number of doses available. After preparation your Spiriva Respimat inhaler will be able to deliver your 60 puffs (30 medicinal doses).
You will need to use this inhaler ONLY ONCE A DAY. Each time you use it take TWO PUFFS.
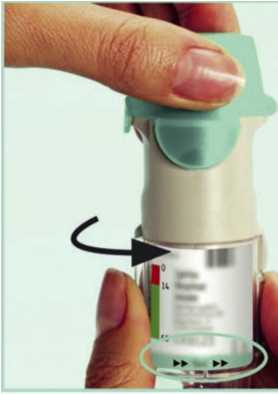
I. Hold the Tiotropium Respimat inhaler upright, with the green cap (A) closed, to avoid accidental release of dose. Turn the base (G) in the direction of the black arrows on the label until it clicks (half a turn).
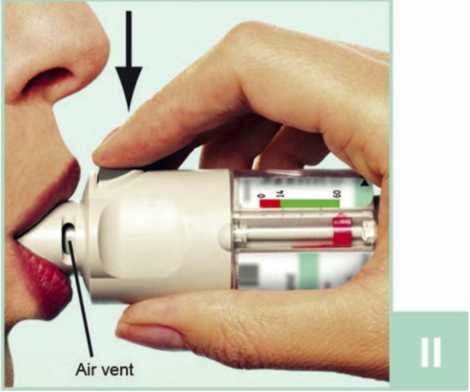
II Open the green cap (A) until it snaps fully open. Breathe out slowly and fully, and then close your lips around the end of the mouthpiece without covering the air vents (C). Point your Tiotropium Respimat inhaler to the back of your throat.
While taking in a slow, deep breath through your mouth, press the dose release button (D) and continue to breathe in slowly for as long as you can. Hold your breath for 10 seconds or for as long as comfortable.
III Repeat steps I and II so that you get the full dose.
You will need to use this inhaler only ONCE A DAY.
Close the green cap until you use your Tiotropium Respimat inhaler again.
If Tiotropium inhaler has not been used for more than 7 days release one puff towards the ground. If Tiotropium Respimat inhaler has not been used for more than 21 days repeat steps 4 to 6 until a cloud is visible. Then repeat steps
4 to 6 three more times.
What if...
|
What if... |
Reason |
What to do |
|
I can’t turn the base easily. |
a) The Tiotropium inhaler is already prepared and ready to use. b) The Tiotropium inhaler is locked after 60 puffs (30 medicinal doses). |
a) The Tiotropium inhaler can be used as it is. b) Prepare and use your new Tiotropium inhaler. |
|
The cap is fully pulled off and apart from the inhaler. |
While opening the cap it was pulled too hard. |
The cap can easily be attached again. |
|
I can’t press the dose release button. |
The clear base has not been turned. |
Turn the clear base until it clicks. (half a turn) |
|
The clear base springs back after I have turned it. |
The clear base was not turned far enough. |
Prepare the Tiotropium inhaler for use by turning the clear base until it clicks, (half a turn) |
|
I can turn the clear base past the point where it clicks. |
Either the dose release button has been pressed, or the clear base has been turned too far. |
With the green cap closed, turn the base until it clicks, (half a turn) |
Clean the mouthpiece including the metal part inside the mouthpiece with a damp cloth or tissue only, at least once a week. Any minor discolouration in the mouthpiece does not affect the performance of your Tiotropium Respimat inhaler.
If necessary, wipe the outside of your Tiotropium Respimat inhaler with a damp cloth.
The Tiotropium Respimat inhaler must not be disassembled after inserting the cartridge and replacing the clear base.
Do not touch the piercing element inside the base.
When to get a new Tiotropium Respimat inhaler

The Tiotropium Respimat inhaler contains 60 puffs (30 medicinal doses). The dose indicator shows approximately how much medication is left. When the pointer enters the red area of the scale, there is, approximately, medication for 7 days left (14 puffs). This is when you need to get a new Tiotropium inhaler prescription.
Once the dose indicator has reached the end of the red scale (i.e. all 30 doses have been used), the Tiotropium Respimat inhaler locks automatically - no more doses can be released. At this point, the base cannot be turned any further.
At the latest, three months after use the Tiotropium Respimat inhaler should be discarded even if not all medication has been used.
Tiotropium 2.5 microgram Respimat®inhalation solution
Ref: 1645/080616/2/B
Patient Information Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Your medicine is called Tiotropium 2.5 microgram, inhalation solution and will be referred to as Tiotropium throughout the rest of this leaflet.
Q What Tiotropium is and what it is used for Q What you need to know before you take Tiotropium Q How to take Tiotropium Q Possible side effects Q How to store Tiotropium Q Contents of the pack and other information
Q What Tiotropium is and what it is used for
Tiotropium helps people who have chronic obstructive pulmonary
disease (COPD) or asthma to breathe more easily. COPD is a long-term lung
disease that causes shortness of breath and coughing. The term COPD
is associated with the conditions chronic bronchitis and emphysema. Asthma
is a long-term disease characterised by airway inflammation and narrowing of
the airways. As COPD and asthma are long-term diseases you should take
Tiotropium every day and not only when you have breathing
problems or other symptoms. When used to treat asthma you should use
Tiotropium in addition to so-called inhaled corticosteroids and
long-acting IJ2 agonists.
Tiotropium is a long-acting bronchodilator that helps to open your airways and makes it easier to get air in and out of the lungs. Regular use of Tiotropium can also help you when you have on-going shortness of breath related to your disease, and will help to minimise the effects of the disease on your everyday life. Daily use of Tiotropium will also help to prevent any sudden, short-term worsening of your COPD symptoms which may last for several days.
For correct dosing of Tiotropium please see section 3. How to take Tiotropium and the instructions for use provided on the other side of the leaflet.
Q What you need to know before you take Tiotropium Please read the following questions carefully.
If you can answer any of these questions with ‘Yes’ please discuss this with your doctor before taking Tiotropium
• are you allergic (hypersensitive) to tiotropium, atropine or similar drugs such as ipratropium or oxitropium?
• are you taking any other medicinal products containing ipratropium or oxitropium?
• are you pregnant, do you think you are pregnant, or are you breast-feeding?
• are you suffering from blurred vision, eye pain and/or red eyes, prostate problems or have difficulty passing urine?
• do you have any kidney problems?
• have you suffered from a myocardial infarction during the last 6 months or from any unstable or life threatening irregular heart beat or severe heart failure within the past year?
• if you are allergic (hypersensitive) to tiotropium, its active ingredient or any of the other ingredients of this medicine (listed in section 6)
• if you are allergic (hypersensitive) to atropine or substances related to it, e.g. ipratropium or oxitropium
Talk to your doctor before taking Tiotropium .
When taking Tiotropium take care not to let any spray enter your eyes. This may result in eye pain or discomfort, blurred vision, seeing halos around lights or coloured images in association with red eyes (i.e. narrow angle glaucoma). Eye symptoms may be accompanied by headache, nausea or vomiting. Wash your eyes in warm water, stop using Tiotropium and immediately consult your doctor for further advice.
If your breathing has got worse or if you experience rash, swelling or itching directly after using your inhaler, stop using it and tell your doctor immediately.
Dry mouth which has been observed with anti-cholinergic treatment may in the long term be associated with dental caries. Therefore, please remember to pay attention to oral hygiene.
Tiotropium is indicated for the maintenance treatment of your chronic obstructive pulmonary disease or asthma. Do not use this medicine to treat a sudden attack of breathlessness or wheezing. Your doctor should have given you another inhaler (“rescue medication”) for this.
Please follow the instructions your doctor has given you.
If you have been prescribed Tiotropium for your asthma it should be added on to inhaled corticosteroids and long-acting IJ2 agonists. Continue taking the inhaled corticosteroids as prescribed by your doctor, even if you feel better.
In case you have suffered from a myocardial infarction during the last 6 months or from any unstable or life threatening irregular heart beat or severe heart failure within the past year, please, inform your doctor. This is important to decide if Tiotropium is the right medicine for you to take.
Do not take Tiotropium more frequently than once daily.
You should also contact your doctor if you feel that your breathing is worsening.
If you have cystic fibrosis, tell your doctor because Tiotropium could make your cystic fibrosis symptoms worse.
Tiotropium is not recommended for children and adolescents under 18 years. Other medicines and Tiotropium
Please tell your doctor or pharmacist if you are taking, or have recently taken, any other medicines, including medicines obtained without a prescription.
In particular, please tell your doctor or pharmacist if you are taking/have taken anticholinergic drugs, e.g. ipratropium or oxitropium.
No interaction side effects have been reported when Tiotropium has been taken with other products used to treat COPD such as reliever inhalers (e.g. salbutamol), methylxanthines (e.g. theophylline), antihistamines, mucolytics (e.g. ambroxol), leukotriene modifiers (e.g. montelukast), cromones, anti-lgE treatment (e.g. omalizumab) and/or inhaled or oral steroids (e.g. budesonide, prednisolone).
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. You should not use this medicine unless specifically recommended by your doctor.
No studies on the effects and the ability to drive and use machines have been performed. In case dizziness or blurred vision occurs the ability to drive and use machinery may be influenced.
Q How to take Tiotropium
Always take this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Tiotropium is for inhalation use only.
The recommended dose for adults is:
Tiotropium is effective for 24 hours so you will need to use Tiotropium only ONCE A DAY, if possible at the same time of the day. Each time you use it take TWO PUFFS.
As COPD and asthma are long-term diseases take Tiotropium every day and not only when you experience breathing problems. Do not take more than the recommended dose.
Tiotropium is not recommended for use in children and adolescents below 18 years due to lack of data on safety and efficacy.
Make sure that you know how to use your Tiotropium Respimat inhaler properly. The instructions for use of the Tiotropium Respimat inhaler are provided on the other side of this leaflet.
If you take more Tiotropium than you should
If you take more Tiotropium than two puffs in one day talk to your doctor immediately. You may be at a higher risk of experiencing a side effect such as dry mouth, constipation, difficulties passing urine, increased heart beat or blurred vision.
If you forget to take Tiotropium
If you forget to take your daily dose (TWO PUFFS ONCE A DAY), don’t worry. Take it as soon as you remember but do not take two doses at the same time or on the same day. Then take your next dose as usual.
Before you stop taking Tiotropium, you should talk to your doctor or your pharmacist. If you stop taking Tiotropium the signs and symptoms of COPD may worsen.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
POM
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Evaluation of the side effects is based on the following frequencies:
Common: may affect up to 1 in 10 people Uncommon: may affect up to 1 in 100 people Rare: may affect up to 1 in 1,000 people
Not known: frequency cannot be estimated from the available data
The side effects described below have been experienced by people taking this medicine and they are listed according to frequency as either common, uncommon, rare or not known
|
Side effect |
Frequency COPD |
Frequency Asthma |
|
Dry mouth: this is usually mild |
Common |
Common |
|
Dizziness |
Uncommon |
Uncommon |
|
Headache |
Uncommon |
Uncommon |
|
Difficulty in sleeping (insomnia) |
Rare |
Uncommon |
|
Irregular heart beat (atrial fibrillation, supraventricular tachycardia) |
Rare |
Not known |
|
Feeling your heartbeat (palpitations) |
Rare |
Uncommon |
|
Faster heart beat (tachycardia) |
Rare |
Not known |
|
Cough |
Uncommon |
Uncommon |
|
Nosebleed (epistaxis) |
Rare |
Not Known |
|
Inflammation of the throat (pharyngitis) |
Uncommon |
Uncommon |
|
Hoarseness (dysphonia) |
Uncommon |
Uncommon |
|
Tightness of the chest, associated with coughing, wheezing or breathlessness immediately after inhalation (bronchospasm) |
Rare |
Uncommon |
|
Constipation |
Uncommon |
Rare |
|
Fungal infections of the oral cavity and throat (oropharyngeal candidiasis) |
Uncommon |
Uncommon |
|
Difficulties swallowing (dysphagia) |
Rare |
Not known |
|
Rash |
Uncommon |
Rare |
|
Itching (pruritus) |
Uncommon |
Rare |
|
Difficulties passing urine (urinary retention) |
Uncommon |
Not known |
|
Painful urination (dysuria) |
Uncommon |
Not known |
|
Seeing halos around lights or coloured images in association with red eyes (glaucoma) |
Rare |
Not known |
|
Increase of the measured eye pressure |
Rare |
Not known |
|
Blurred vision |
Rare |
Not know |
|
Inflammation of the larynx (laryngitis) |
Rare |
Not known |
|
Heart burn (gastrooesophageal reflux disease) |
Rare |
Not Known |
|
Dental caries |
Rare |
Not known |
|
Inflammation of the gums (gingivitis) |
Rare |
Rare |
|
Inflammation of the tongue (glossitis) |
Rare |
Not known |
|
Inflammation of the mouth (stomatitis) |
Not known |
Rare |
|
Serious allergic reaction which causes swelling of the mouth and face or throat (angioneurotic oedema) |
Rare |
Rare |
|
Nettle rash (urticaria) |
Rare |
Rare |
|
Infections or ulcerations of the skin |
Rare |
Not known |
|
Dryness of the skin |
Rare |
Not known |
|
Hypersensitivity, including immediate reactions |
Not known |
Rare |
|
Infections of the urinary tract |
Rare |
Not known |
|
Depletion of body water (dehydration) |
Not known |
Not known |
|
Inflammation in sinuses (sinusitis) |
Not known |
Not known |
|
Blockage of intestines or absence of bowel movements (intestinal obstruction, including ileus paralytic) |
Not known |
Not known |
|
Feeling sick (nausea) |
Not known |
Not known |
|
Severe allergic reaction (anaphylactic reaction) |
Not known |
Not known |
|
Swelling of joint |
Not known |
Not known |
Immediate allergic reactions such as rash, nettle rash (urticaria), swelling of the mouth and face or sudden difficulties in breathing (angioneurotic oedema) or other hypersensitivity reactions (such as sudden reduction of your blood pressure or dizziness) may occur individually or as part of severe allergic reaction (anaphylactic reaction) after administration of Tiotropium. If any of these occur, please consult your doctor immediately
In addition, in common with all inhaled medicines, some patients may experience an unexpected tightness of the chest, coughing, wheezing or breathlessness immediately after inhalation (bronchospasm).
If you get any side effects, talk to your doctor or pharmacist.This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (Tel: Freephone 0808 100 3352 or Website: www.mhra.aov.uk/vellowcard1. By reporting side effects you can help provide more information on the safety of this medicine.
Q How to store Tiotropium
Keep out of the sight and reach of children.
Do not use Tiotropium after the expiry date which is stated on the carton and on the inhaler label. The expiry date refers to the last day of the month. Tiotropium Respimat inhaler should be discarded at the latest 3 months after first use (see Instructions for Use overleaf).
Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
If your medicine becomes discoloured or show any other signs of deterioration, ask your pharmacist who will advise you what to do.
Q Contents of the pack and other information What Tiotropium contains:
The active substance is tiotropium. The delivered dose is 2.5 micrograms tiotropium (as bromide monohydrate) per puff (2 puffs comprise one medicinal dose).
The other ingredients are: Benzalkonium chloride, disodium edetate, purified water, and hydrochloric acid 3.6% for pH adjustment.
What Tiotropium looks like and contents of the pack
The cardboard carton contains one inhaler and one aluminium cylindrical cartridge containing a clear colourless solution for inhalation. A plastic cap is present on both ends of the inhaler: a green cap covering the mouth piece; and a transparent cap where the cartridge is to be inserted. The inhaler has an indicator on the side showing the number of doses left.
Single pack: 1 Respimat inhaler and 1 cartridge, providing 60 puffs (30 medicinal doses)
Manufacturer and Licence Holder
This medicine is manufactured by Boehringer Ingelheim Pharma GmbH &
Co. KG, Binger Strasse 173, D-55216 Ingelheim am Rhein, Germany and is procured from within the EU. Product Licence Holder: Lexon UK Limited, Unit 18 Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 ORE.
If you have any questions or are not sure about anything, ask your doctor or pharmacist. They will have additional information about this medicine and will be able to advise you.
Respimat is a registered trademark of Boehringer ingelheim Pharma GmbH & Co. KG.
PL 15184/1645 - Tiotropium 250 microgram Respimat Inhalation solution
Leaflet revision date: 08/06/16
Blind or partially sighted? Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Instructions for Use
Tiotropium Respimat Inhaler
How to use your Tiotropium Respimat Inhaler
This leaflet explains how to use and care for your Tiotropium Respimat inhaler. Please read and carefully follow these instructions. See also section 3. How to take Tiotropium on the other side of this leaflet.
The Tiotropium Respimat inhaler releases medication slowly and gently, making it easy to inhale it into your lungs.
The Tiotropium Respimat inhaler enables you to inhale the medicine contained in a cartridge. The full cartridge provides 60 puffs (30 medicinal doses). You will need to use this inhaler only ONCE A DAY, if possible at the same time of the day. Each time you use it take TWO PUFFS.
There is enough medicine for 30 days when it is used according to the directions for use. In the box you will find the Tiotropium Respimat inhaler and the Tiotropium cartridge. Before the Tiotropium Respimat inhaler is used for the first time, the cartridge provided must be inserted.
Ca p (A)
Mouthpiece (B) Air vent (C)
Dose release button (D)
Safety catch (E)
Clear base (G)
Piercing element (I)
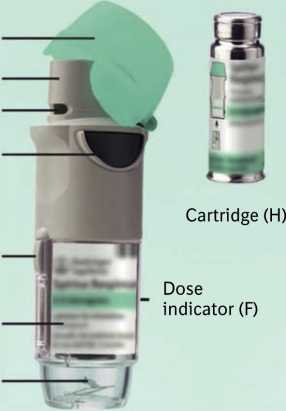

Tiotropium Respimat inhaler and the Tiotropium cartridge
1) Inserting the cartridge
The following steps 1-6 are necessary before first use:
3. Replace the clear base (G). Do not remove the clear base again.
2) To prepare the Tiotropium Respimat inhaler for first-time use
4. Hold the Tiotropium Respimat inhaler upright, with the green cap (A) closed. Turn the base (G) in the direction of the red arrows on the label until it clicks (half a turn).
1 With the green cap (A) closed, press the safety catch (E) while pulling off the clear base (G).

5. Open the green cap (A) until it snaps fully open.

6 Point the Tiotropium Respimat inhaler towards the ground. Press the dose release button (D). Close the green cap (A).
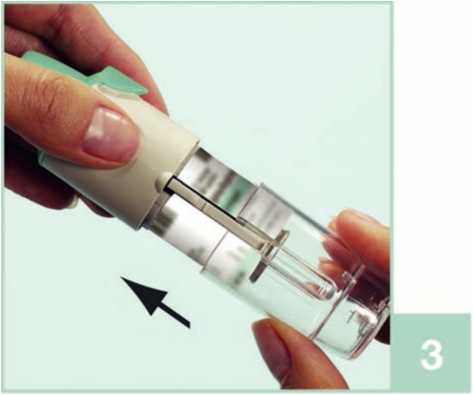
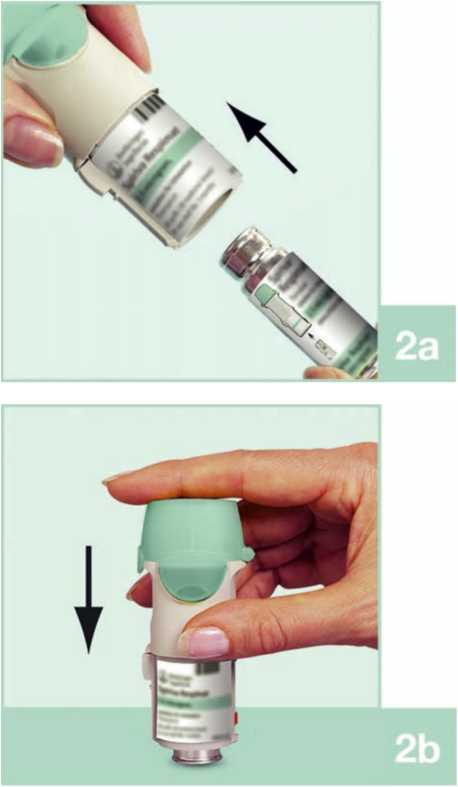
2. Take the cartridge (H) out of the box. Push the narrow end of the cartridge into the inhaler until it clicks into place. The cartridge should be pushed firmly against a firm surface to ensure that it has gone all the way in (2b).
The cartridge will not be flush with the inhaler, you will still see the silver ring of the lower end of the cartridge.
Do not remove the cartridge once it has been inserted into the inhaler
Then repeat steps 4, 5 and 6 three more times to ensure the inhaler is prepared for use.
Your Tiotropium Respimat inhaler is now ready to use.
These steps will not affect the number of doses available. After preparation your Tiotropium Respimat inhaler will be able to deliver your 60 puffs (30 medicinal doses).
Ref: 1645/080616/2/B Page 3