Vancomycin 500 Mg Powder For Concentrate For Solution For Infusion
K0000
K0000

Package leaflet: Information for the user
Vancomycin 500mg and 1000mg
Powder for Concentrate for Solution for Infusion
Vancomycin
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
T| What Vancomycin is and what it is used for
3] What you need to know before you use Vancomycin
31 How to use Vancomycin "4 Possible side effects 3] How to store Vancomycin 3| Contents of the pack and other information
j What Vancomycin is and what it is used for
How does the medicine work?
Vancomycin contains the active ingredient vancomycin, which is an antibiotic. Antibiotics help your body fight infections. Vancomycin works by eliminating certain bacteria that cause infections.
What is the medicine used for?
Vancomycin is used for the treatment of serious infections caused by certain bacteria, such as infections of the bones, lung infections, skin and muscle (soft tissue) infection, infection of the valves or lining of the heart.
3) What you need to know before you use Vancomycin Do not use Vancomycin
• If you are allergic (hypersensitive) to vancomycin (an allergic reaction presents with symptoms such as a rash, itching, swelling or breathing difficulties after receiving this medicine).
Warnings and precautions
Talk to your doctor, pharmacist or nurse
before using Vancomycin:
• if you have kidney problems
• if you are hard of hearing
• if you are pregnant or planning to become pregnant
• if you are breast-feeding
• if you are elderly, over the age of 60.
Your doctor may perform several tests to see if your kidneys and liver are working properly.
If you are elderly or have kidney problems your doctor may also perform regular tests on your hearing and measure the amount of vancomycin in your blood.
Deafness, transitory or permanent, which may be preceded by noises in ears, can occur in patients with prior deafness, who have received excessive doses, or who receive treatment with another substance toxic to hearing. To reduce this risk, blood levels should be checked periodically and periodic testing of hearing function is recommended.
Rapid injection of vancomycin may cause low blood pressure, shock and rarely cardiac arrest. Stopping the infusion usually results in a prompt cessation of the reactions.
Injection site pain, inflammation of the vein wall and blood clotting can occur and is occasionally severe, slow administration also reduces these side effects.
Other medicines and Vancomycin
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
The following medicines may interact with Vancomycin:
• anaesthetic agents
• medicine for muscle relaxation
• medicines for infections caused by bacteria (e.g. polymyxin B, colistin, bacitracin, aminoglycosides)
• medicine for fungal infection (amphotericin B)
• medicine for tuberculosis (viomycin)
• medicine for cancer (cisplatin).
Drugs that can affect the kidneys and hearing
If you receive vancomycin together with other drugs that may be harmful to the kidneys and the hearing (e.g. aminoglycoside antibiotics), the harmful effect may be increased. In such cases, careful and regular monitoring of renal function and hearing is necessary.
Anaesthetic agents
Use of anaesthetic agents increase the risk of side effects of vancomycin such as hypotension, rash, hives and itching.
Muscle relaxants
If you receive muscle relaxants (e.g. succinylcholine), together with vancomycin the effect of these may be enhanced or extended.
If you are allergic to another antibiotic called teicoplanin you may also be allergic to vancomycin. Please tell your doctor.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Vancomycin should be given during pregnancy and breast-feeding only if clearly needed. The doctor may decide that you should stop breast-feeding.
Driving and using machines
Vancomycin has no or very little effect on your ability to drive and use machines.
_3| How to use Vancomycin
This medicine will always be administered by a healthcare professional.
Your doctor will decide at which rate and how long you will be given the medicine. The dose your doctor gives you will depend on your age, weight, kidney functions and the severity of the infection.
The amount of medicine in your blood will usually be measured at regular intervals. Your doctor may also do blood tests to check your kidneys and tests checking your ears, especially if you are elderly.
• The medicine is given into a vein, usually in your arm, slowly over at least one hour.
Dosage in adults and children above 12 years by infusion:
The usual dosage is 2000mg daily in two or four doses (500mg every 6 hours or 1000mg every 12 hours) or will be calculated depending on the body weight.
Dosage in children from one month to 12 years:
The dosages will be calculated depending on their body weight.
The usual intravenous dosage is 10mg/kg per dose given every six hours (total daily dosage 40mg/kg of body weight). Each dose is administered over a period of at least 60 minutes.
Newborn infants (full-term):
0-7 days of age: A starting dose of 15mg/kg, followed by 10mg/kg every 12 hours.
7-30 days of age: A starting dose of 15mg/kg, followed by 10mg/kg every 8 hours.
Each dose is administered over 60 minutes.
Dosage in patients with impaired kidney function and the elderly by infusion:
Your doctor may reduce the dose depending on how well your kidneys function.
continued over page
continued top of next column
Vancomycin 500mg and 1000mg Powder for Concentrate for Solution for Infusion
Vancomycin
The following information is intended for healthcare professionals only.
Vancomycin powder for concentrate for solution for infusion is for single use only and any unused solution should be discarded.
The powder must be reconstituted and the resulting concentrate must then be immediately diluted further prior to use.
Preparation of the reconstituted concentrate
Dissolve the content of each 500mg vial in 10ml of sterile water for injections.
Dissolve the content of each 1000mg vial in 20ml of sterile water for injections.
One ml of reconstituted solutions contains 50mg vancomycin. pH of the reconstituted solutions is 2.5 to 4.5.
The solution should be clear colourless to pale yellow and free from fibre and visible particulate matters.
Preparation of final diluted solution for infusion
Reconstituted concentrate containing 50mg/ml of vancomycin should be further diluted depending on the method of administration.
Suitable diluents are: 5% Glucose Injection, 0.9% Sodium Chloride Injection, 0.9% Sodium Chloride and 5% Glucose Injection or Ringer acetate Injection.
Intermittent infusion
Reconstituted concentrate containing 500mg of vancomycin (50mg/ml) must be diluted further with at least 100ml diluent.
Reconstituted concentrate containing 1000mg of vancomycin (50mg/ml) must be diluted further with at least 200ml diluent.
The concentration of vancomycin in solution for infusion should not exceed 5mg/ml.
The desired dose should be administrated slowly by intravenous infusion at a rate of no more than 10mg/ minute, for at least 60 minutes or even longer.
Before administration, the reconstituted and diluted solutions should be inspected visually for particulate matter and discolouration. Only clear and colourless solution free from particles should be used.
Do not tamper with the bag/bottle. Follow the doctor's instructions.
If you receive too much Vancomycin
Your doctor monitors the amount of Vancomycin you receive. If blood tests and other tests show that you have too much in your body, the amount of Vancomycin will be reduced or treatment may be interrupted or stopped. The level remaining in your blood will be lowered.
If you have any further questions on the use of this medicine, ask your doctor or other healthcare professional.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you get:
• any sudden wheeziness, difficulty in breathing, redness on the upper part of the body, rash or itching. These may be signs of a serious allergic reaction (anaphylactic shock). This side effect occurs rarely (may affect up to 1 in 1,000 people).
• fever, severe rash, joint pain, enlarged lymph nodes and inflammation of one or more internal organs such as liver leading to abdominal pain, yellowing of the skin and the whites of the eyes and/or heart, lungs and kidneys; with changes to your blood counts, particularly white blood cells called eosinophils. These may be signs of
a multi-organ sensitivity disorder called DRESS syndrome. The frequency of this side effect is not known (cannot be estimated from the available data).
Other possible side effects include:
Common side effects (may affect up to 1 in 10 people)
• fall in blood pressure
• breathlessness, noisy breathing (a high pitched sound resulting from turbulent air flow in the upper airway)
• pain, redness and swelling at the site where the needle is inserted in the vein
• rash and inflammation of the lining of the mouth, itching, itching rash, hives
• kidney problems which may be detected primarily by blood tests
• redness of upper body and face, inflammation of a vein
• vein wall inflammation with blood clotting (thrombophlebitis)
• skin reactions such as rashes, swelling, itching or hives.
Uncommon side effects (may affect up to 1 in
100 people)
• temporary or permanent loss of hearing.
Rare side effects (may affect up to 1 in 1,000 people)
• allergic reactions
• increase or decrease in some of the white cells in the blood, decrease in platelets (blood cells responsible for blood clotting)
• ringing in your ears, dizziness
• blood vessel inflammation (vasculitis)
• nausea (feeling sick)
• diarrhoea
• pain in the chest and back muscles
• fever, chills
• inflammation of the kidneys
• acute kidney failure.
Very rare side effects (may affect up to 1 in
10,000 people)
• sudden onset of severe allergic skin reaction with skin flaking blistering or peeling skin (Stevens-Johnson Syndrome). This may be associated with a high fever and joint pains
• cardiac arrest
• inflammation of the bowel which causes abdominal pain and diarrhoea, which may contain blood.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via
United Kingdom
Yellow Card Scheme Tel: 0808 100 3352
Website: www.mhra.gov.uk/yellowcard Ireland
HPRA Pharmacovigilance
Earlsfort Terrace, IRL - Dublin 2
Tel: +353 1 6764971 Fax: +353 1 6762517
Website: www.hpra.ie
e-mail: medsafety@hpra.ie
By reporting side effects you can help provide more information on the safety of this medicine.
How to store Vancomycin
Your doctor will be responsible for storing the medicine.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial label and outer carton after EXP. The expiry date refers to the last day of that month.
Powder as packaged for sale:
Store below 25 °C.
Keep the vial in the outer carton in order to protect from light.
The stability of the reconstituted concentrate and further diluted product is stated below in the additional information for medical or healthcare professionals.
Do not throw away medicines via wastewater or household waste. Ask your phamacist how to throw away medicines you no longer use. These measures will help protect the environment.
Contents of the pack and other information
What Vancomycin contains:
The active ingredient is vancomycin.
- Vancomycin 500mg powder for concentrate for solution for infusion: Each vial contains 500mg vancomycin (as vancomycin hydrochloride) equivalent to 500,000 IU.
- Vancomycin 1000mg powder for concentrate for solution for infusion: Each vial contains 1000mg vancomycin (as vancomycin hydrochloride) equivalent to 1,000,000 IU.
What Vancomycin looks like and contents of the pack:
Vancomycin 500mg powder for concentrate for solution for infusion:
- A white to cream coloured porous cake powder in a clear glass vial with a grey flip-off cap.
Pack size: 1 vial.
Vancomycin 1000mg powder for concentrate for solution for infusion:
- A white to cream coloured porous cake powder in a clear glass vial with a green flip-off cap.
Pack size: 1 vial.
The medicine is a powder that has to be dissolved before you receive it.
Marketing Authorisation Holder:
Actavis Group PTC ehf.
Reykjavikurvegi 76-78 220 HafnarfjorSur Iceland
Manufacturer
Xellia Pharmaceuticals ApS Dalslandsgade 11 2300 Copenhagen S Denmark
This leaflet was last revised in April 2016
If you would like a leaflet with larger text, please contact UK: 01271 385257 IE: 1890 333231
continued top of next column AAAI0409
^actav/s
Actavis, Barnstaple, EX32 8NS, UK Actavis Ireland, Euro HS, Little Island, Cork
Shelf-life of reconstituted concentrate:
The reconstituted concentrate should be further diluted immediately after reconstitution.
Shelf-life of diluted product:
From a micobiological point of view, the product should be used immediately.
In patients with impaired renal function
the dosage must be adjusted. Serum levels of vancomycin should be monitored regularly. For most patients with impaired renal function the following nomogram can be used to determine the dosage needed. The total daily dose of vancomycin (in mg) should be about 15 times the glomerular filtration rate (in ml/min). The starting dose should always be at least 15mg/kg. The nomogram is not valid for functionally anephric patients on dialysis.
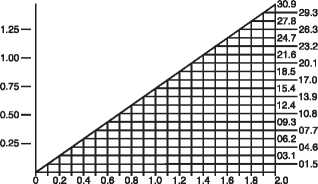
Vancomycin Dosing (mg/kg/24hr)
CREATININE CLEARANCE (ml/min/kg)
If the creatinine clearance is not available, the following formula may be applied to calculate the creatinine clearance from the patient's age, sex and serum creatinine:
Men: Weight (kg) x 140 - age (years)
72 x serum creatinine (mg/100ml)
Women: 0.85 x value calculated by the above formula.
continued top of next column
AAAI0409
$actav/s
Actavis, Barnstaple, EX32 8NS, UK Actavis Ireland, Euro HS, Little Island, Cork