Vancomycin 500 Mg Powder For Concentrate For Solution For Infusion
PACKAGE LEAFLET: INFORMATION FOR THE USER
Vancomycin 500 mg, Powder for concentrate for solution for infusion Vancomycin 1000 mg, Powder for concentrate for solution for infusion
Vancomycin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What is Vancomycin and what it is used for
2. What you need to know before you use Vancomycin
3. Using Vancomycin
4. Possible side effects
5. How to store Vancomycin
6. Contents of the pack and other information
Vancomycin is an antibiotic which helps your body fight infections by eliminating certain bacteria that cause infections. Vancomycin is used for the treatment of the following serious infections:
Infections of the bones, lung infections, skin and muscle (soft tissue) infection and infection of the valves or lining of the heart.
Vancomycin can also be used before some types of surgery to prevent possible infections of the lining of the heart.
Vancomycin may also be administered orally in the treatment of:
• inflammation of the lining of the small and large intestine with damage to the mucosa (pseudomembranous colitis) caused by a certain type of bacteria (Clostridium difficile), as a result of treatment with antibiotics;
• inflammation of the lining of the small and large intestine (enterocolitis) as a result of a certain type of bacteria (Staphylococci).
Do not use Vancomycin
• If you are allergic (hypersensitive) to vancomycin or any other ingredients of this medicine (listed in Section 6).
Tell your doctor if you have had some problems with this product or with others in the past.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Vancomycin.
Before treatment with vancomycin, make sure your doctor knows your medical history, especially:
• If you have kidney problems
• If you have difficulty hearing
• If you are over 65 years old
The rapid injection of Vancomycin can cause low blood pressure, shock, and rarely, cardiac arrest. Stopping the infusion usually results in immediate cessation of these reactions.
Injection site pain, inflammation of the vein wall and blood coagulation may occasionally occur in severe form; slow administration can also reduce these side effects.
If you are allergic to another antibiotic called teicoplanin, you may also be allergic to vancomycin. Please tell your doctor.
If you suffer from renal (kidney) impairment or receive treatment simultaneously with other substances that are toxic to the kidney, the possibility of developing toxic effects is very high. Your doctor can perform several tests to see if your kidneys and liver are working properly.
If you are elderly or have kidney problems, your doctor may also perform regular tests to your hearing and measure the amount of vancomycin in the blood.
Transient or permanent hearing loss, which may be preceded by noises in the ears, can occur in patients with prior deafness treated with excessive doses, or in concomitant treatment
with other toxic substances to the ear. To reduce this risk, blood levels should be determined periodically and periodic tests for hearing function are recommended.
Prolonged use of Vancomycin may result in over-abundance of resistant organisms, so your doctor will monitor you.
Other medicines and Vancomycin
Tell your doctor, pharmacist or nurse if you are taking or have recently taken, or might take any other medicines. Moreover, do not take any new medicine without consulting your doctor.
The following medicines may react with vancomycin if you take them at the same time, such as medicines for the treatment of:
• infections caused by bacteria (streptomycin, neomycin, gentamicin, kanamycin, amikacin, bacitracin, tobramycin, polymixin B, colistin),
• tuberculosis (viomycin),
• fungal infections (amphotericin B),
• cancer (cisplatin) and
• medicines for muscle relaxation during anaesthesia,
• anaesthetic agents (if you are going to have general anaesthesia).
Your doctor may need to test your blood and adjust the dosage if vancomycin is given simultaneously with other medicines.
Pregnancy
If you are pregnant, think you may be pregnant or plan to become pregnant, tell your doctor before taking this medicine. Vancomycin should be given during pregnancy and breast-feeding only if clearly needed. Your doctor will decide if you should take Vancomycin.
Breast-feeding
Tell your doctor if you are breastfeeding, since Vancomycin passes into breast milk. Your doctor will decide whether vancomycin is really necessary or whether you should stop breastfeeding.
Driving and using machines
Vancomycin has no or negligible effect on the ability to drive and use machines.
3. How to use Vancomycin
This medicine will always be administered by a healthcare professional. Your doctor will decide at which rate and how long you will be given the medicine. The dose your doctor gives you will depend on your age, weight, kidney function and the severity of the infection.
The amount of medicine in your blood will usually be measured at regular intervals. Your doctor may also do blood tests to check your kidneys and tests to check your ears, especially if you are elderly.
The medicine is given into a vein, usually in your arm, slowly over at least one hour.
Dosage
The dose given to you will take into account your age, the type of infection you have, the functioning of your kidneys, your hearing, and other medicines you may be taking.
The following information is intended only for doctors and healthcare professionals.
This text is an excerpt from the Summary of Product Characteristics to help in the administration of Vancomycin.
In assessing the appropriateness of using in a particular patient, the doctor should be familiar with the Summary of Product Characteristics.
Preparation of reconstituted solution
Dissolve the contents of each 500 mg vial in 10 ml sterile water for injections.
Dissolve the contents of each 1000 mg vial in 20 ml sterile water for injections.
One ml of reconstituted solution contains 50 mg of vancomycin.
For oral administration, the reconstituted solutions containing 500 mg and 1000 mg of vancomycin can be diluted in 30 ml of water and given to the patient or administered through a naso-gastric tube.
Appearance of the reconstituted solution
After reconstitution, the solution is clear and colourless to slightly brownish yellow and has no visible particles.
Preparation of the Anal diluted solution for infusion
The reconstituted solution containing 50 mg/ml of vancomycin should be further diluted.
Suitable diluents are:
• Glucose 5% solution for injection
• Sodium chloride 0.9% solution for injection
• 5% Glucose Injection with 0.9% Sodium Chloride Injection
• Ringer's Lactate Injection
Intermittent infusion:
The reconstituted solution containing 500 mg of vancomycin (50 mg/ml) should first be diluted with at least 100 ml of solvent (to a concentration of 5 mg/ml).
The reconstituted solution containing 1000 mg of vancomycin (50 mg/ml) should first be diluted with at least 200 ml of solvent (to a concentration of 5 mg/ml).
The concentration of vancomycin in the infusion solution should not exceed 5 mg/ml.
The desired dose should be slowly administered intravenously at a rate not exceeding 10 mg/min for at least 60 minutes.
Continuous infusion:
Should only be used if treatment with an intermittent infusion is not possible. Dilute 1000 mg to 2000 mg of dissolved vancomycin in sufficient solvent (mentioned above) and administer as an infusion "drip", so that the patient receives the prescribed daily dose in 24 hours.
Appearance of the diluted solution
After dilution, the solution is clear, free from foreign particles.
Before administration, the reconstituted and diluted solutions should be inspected visually for particles and discoloration. Only clear, colourless solutions free of particles should be used.
Shelf life of the reconstituted solution:
For intravenous use, the reconstituted solution should be diluted immediately after preparation.
For oral use, the reconstituted solution with purified water for oral administration is stable when stored at 2-8 °C for 48 hours.
Adults and children over 12 years:
The usual dose is 2000 mg per day in 2 or 4 doses.
Children 1 month to 12 years: The dosage will be calculated depending on their body weight.
The usual intravenous dosage is 10mg/kg per dose given every six hours (total daily dosage 40mg/kg of body weight).
Newborn infants (full-term):
0-7 days of age: A starting dose of 15 mg/kg, followed by 10 mg/kg every 12 hours.
7-30 days of age: A starting dose of 15 mg/kg, followed by 10 mg/kg every 8 hours.
Patients with impaired renal function, elderly
and preterm infants: The doctor will reduce the dose or increase the interval between two doses.
Oral administration Adults and elderly
The usual daily dose is 500 mg divided into three or four administrations for 7 to 10 days. The total daily dose should not exceed 2000 mg.
Children
The usual daily dose is 40 mg/kg divided into three or four administrations for 7 to 10 days. The total daily dose should not exceed 2000 mg.
During treatment you may need to have blood and urine tests and possibly hearing tests for signs of any side effects.
Duration of treatment
The duration of treatment depends on the infection, and can last several weeks.
If you receive more Vancomycin than you should
As this product will be given to you while you are in the hospital, it is unlikely that you will be given too much. However, tell your doctor or nurse immediately if you have any concerns.
If you have further questions about using this medicine, ask your doctor, pharmacist or nurse.
Side effects of unknown frequency and single reported cases:
• syndrome that may cause a rash, fever, inflammation of internal organs, and characteristic abnormalities of your blood (so called "DRESS"),
• sudden formation of pustules within large swollen areas (so called "AGEP"),
• acute tubular necrosis.
Severe anaphylactoid reactions are possible during or a short time after fast intravenous infusion. The reactions disappear after the infusion is stopped.
Ototoxicity has primarily been reported in patients given high doses, or concomitant treatment with other ototoxic medicinal products, or with pre-existing reduction in kidney function or hearing.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via:
UK: the Yellow Card Scheme at: www.mhra.gov.uk/yellow card.
Ireland: HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2;
Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most common side effects are pain, swelling and inflammation of veins at the infusion site and pseudo-allergic reactions when intravenous infusion of Vancomycin is carried out too fast.
Stop taking the medicine immediately and seek medical attention if any of the following signs of allergic reaction occur:
• hives (nettle rash), swelling of the face, lips, tongue or throat, difficulty breathing or swallowing, dizziness.
If you think you have any of the following side effects or symptoms, tell your doctor as soon as possible:
Common side effects (may occur in fewer than 1 in 10 patients):
• decrease in blood pressure
• thrombophlebitis
• dyspnoea
• stridor
• exanthema and mucosal inflammation
• pruritus
• urticaria
• redness of the upper body and face, pain and spasm of the chest and back muscles
• renal insufficiency manifested primarily by increased blood concentrations of creatinine or urea
Uncommon side effects (may occur in fewer than 1 in 100 patients):
• temporary or permanent hearing loss
Rare side effects (may occur in fewer than 1 in 1,000 patients):
• thrombocytopenia, neutropenia, agranulocytosis, eosinophilia, anaphylactic reactions, hypersensitivity reactions
• tinnitus, dizziness
• vasculitis
• fever, shivering
• nausea, diarrhoea
• interstitial nephritis, acute renal failure
Very rare side effects (may occur in fewer than 1 in 10,000 patients):
• exfoliative dermatitis
• Stevens-Johnson syndrome
• Lyell's syndrome
• IgA induced bullous dermatitis
• cardiac arrest
• pseudomembranous enterocolitis
Before reconstitution: Store below 25°C.
Keep the vial in the outer carton in order to protect from light.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date printed on the label and carton. The expiry date refers to the last day of that month.
Do not use this medicine if you notice that the solution is cloudy or there are particles in suspension.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
What is the composition of Vancomycin
• The active substance is vancomycin (as hydrochloride).
Vancomycin 500 mg: Each vial contains 500 mg of vancomycin equivalent to 525,000 IU.
Vancomycin 1000 mg: Each vial contains 1000 mg of vancomycin equivalent to 1,050,000 IU.
What Vancomycin looks like and contents of the pack
Vancomycin 500 mg comes in the form of a white or slightly brownish powder in clear glass vials with a rubber stopper and orange aluminium cap. Vancomycin 1000 mg comes in the form of a white or slightly brownish powder, in clear glass vials with a rubber stopper and white aluminium cap.
Each package contains 1, 5, 10 or 20 vials.
Before use, the powder is dissolved and diluted with an intravenous liquid, obtaining a solution which will be administered to you slowly into a vein ("drip"), by a doctor or nurse.
Each 500 mg vial contains 512.57 mg of vancomycin hydrochloride, equivalent to 500 mg of vancomycin. After reconstitution with 10 ml of water for injections is obtained a solution with a concentration of 50 mg/ml, and after further dilution a solution with a concentration of 5 mg/ml is obtained.
Each 1000 mg vial contains 1025.16 mg of vancomycin hydrochloride, equivalent to 1000 mg of vancomycin. After reconstitution with 20 ml of water for injections is obtained a solution with a concentration of 50 mg/ml, and after further dilution a solution with a concentration of 5 mg/ml is obtained.
Marketing Authorisation Holder
Quimedical - Produtos Farmaceuticos, Lda. Edificio Azevedos, Estrada Nacional 117-2, Alfragide, 2614-503 Amadora, Portugal
Manufacturer
Sofarimex - Industria Quimica e Farmaceutica, S.A. Av. das Industria, Alto de Colaride, 2735-213 Cacem, Portugal
This leaflet was last revised in March 2016
Shelf life of diluted solution:
Chemical and physical in-use stability has been demonstrated:
• for a period of 24 hours at 25 °C after reconstitution and further dilution with sodium chloride 9 mg / ml (0.9%) or glucose solution 50 mg / ml (5%);
• for a period of 96 hours when stored at 2-8°C after reconstitution and further dilution with sodium chloride 9 mg / ml (0.9%) or glucose solution 50 mg / ml (5%), or Ringer's lactate solution or with sodium chloride 9 mg / ml (0.9%) + glucose 50 mg / ml (5%).
Patients with renal impairment:
Dosage adjustments must be made to avoid toxic serum levels, hence serum levels of vancomycin should be monitored regularly.
For most patients with impaired renal function the following nomogram, based on creatinine clearance values, can be used to determine the dose needed.
The nomogram is not valid for functionally anephric patients on dialysis.
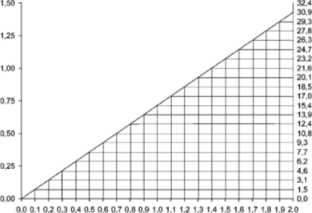
If serum creatinine level alone is available, the following formula may be applied to calculate the creatinine clearance:
Men: Weight (kg) x (140 - age (years))
72 x serum creatinine (mg/100ml)
Women: 0.85 x value calculated by the above formula.
Where possible, the creatinine clearance should always be determined.
Patient on hemodialysis
Serum levels of vancomycin should be monitored regularly.
Patients with anuria (with practically no renal function) on dialysis should receive a dose of 15 mg/kg body weight to achieve therapeutic serum levels promptly. The maintenance doses are 1.9 mg/kg body weight per 24 hours.
Since individual maintenance doses of 250 mg to 1 g are convenient, adult patients with markedly impaired renal function may receive a maintenance dose of 250 - 1000 mg at intervals of several days instead of a daily dose. In anuria, a dose of 1g every 7-10 days has been recommended.
If polysulfone membranes are used in haemodialysis (high flow dialysis), the half-life of vancomycin is reduced. Additional maintenance doses in patients undergoing regular haemodialysis may be required.
CREATING CLEARANCE (mlfm.iWg)