Water For Injections
Out of date information, search another8-9402-01-010-2_v1-11:Layout 1 2015-05-28
4:56 PM
Page
1
Package leaflet: Information for the user
GlucaGen® HypoKit 1 mg
Powder and solvent for solution for injection
Glucagon
Read all of this leaflet carefully before you are given this injection because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What GlucaGen® HypoKit is and what it is used for
2. What you need to know before you use GlucaGen® HypoKit
3. How to use GlucaGen® HypoKit
4. Possible side effects
5. How to store GlucaGen® HypoKit
6. Contents of the pack and other information
7. Additional information for medical professionals
1. What GlucaGen® HypoKit is and what it is used for
GlucaGen® HypoKit contains the active substance "glucagon”.
GlucaGen® HypoKit is for immediate, emergency use for children and adults with diabetes who use insulin. It is used when they have passed out (become unconscious) because of very low blood sugar. This is called "severe hypoglycaemia”. GlucaGen® HypoKit is used when they are not able to take sugar by mouth.
Glucagon is a natural hormone, which has the opposite effect of insulin in the human body.
It helps the liver to change something called "glycogen” into glucose (sugar). Glucose is then released into the blood stream - this makes the blood sugar level rise.
For medical professionals: See section 7.
2. What you need to know before you use GlucaGen® HypoKit
Important information
• Make sure that your family members, people you work with or close friends know about GlucaGen® HypoKit. Tell them that if you pass out (become unconscious) they should use GlucaGen® HypoKit straight away.
• Show your family members and others where you keep this kit and how to use it. They must act quickly - if you are unconscious for a period of time it may be harmful. It is important they are trained and know how to use GlucaGen® HypoKit before you need it.
• The syringe does not contain GlucaGen®. The water in the syringe must be mixed with the compacted GlucaGen® powder in the vial before the injection. Tell your family members and others to follow the instructions in section 3: How to use GlucaGen® HypoKit.
• Any mixed GlucaGen® that is not used must be thrown away.
• After using GlucaGen® HypoKit, you or someone else must contact your doctor or
a healthcare provider. You need to find out why you had very low blood sugar and how to avoid it happening again.
Do not use GlucaGen® HypoKit if:
• you are allergic to glucagon or any of the other ingredients of this medicine (listed in section 6).
• you have a tumour in your adrenal gland.
If any of these apply, do not use GlucaGen® HypoKit.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you use GlucaGen® HypoKit.
GlucaGen® will not work properly if:
• you have been fasting for a long time
• you have low levels of adrenaline
• you have low blood sugar caused by drinking too much alcohol
• you have a tumour that releases glucagon or insulin If any of these apply, talk to your doctor or pharmacist.
Other medicines and GlucaGen®
The following medicines can affect the way GlucaGen® HypoKit works:
• insulin - used to treat diabetes
• indomethacin - used to treat joint pain and stiffness
The following medicines may be affected by GlucaGen® HypoKit:
• warfarin - used to prevent blood clots. GlucaGen® may increase the blood-thinning effect of warfarin.
• beta-blockers - used to treat high blood pressure and irregular heart beat.
GlucaGen® HypoKit may increase blood pressure and pulse, this will only last a short time.
If any of the above apply to you (or you are not sure), talk to your doctor or pharmacist before having GlucaGen® Hypokit.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy and breast-feeding
If you experience very low blood sugar, when you are pregnant or breast-feeding, think you might be pregnant or are planning to have a baby, you can use GlucaGen® HypoKit.
Ask your doctor or pharmacist for advice before taking any medicine, if you are pregnant.
4. Inject the dose under the skin or into a muscle.
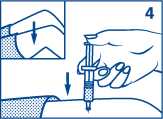
5. Turn the unconscious person on their side to prevent choking.
6. Give the person a high sugar snack like sweets, biscuits or fruit juice as soon as he or she regains consciousness and is able to swallow. The high sugar snack will stop the low blood sugar happening again.
After using GlucaGen® HypoKit, you or someone else must contact your doctor or a healthcare provider. You need to find out why you had very low blood sugar and how to avoid it happening again.
How much to use
The recommended dose is:
• Adults: inject all of the medicine (1 ml) - this is marked as " 1 ” on the syringe.
• Children younger than 8 years or children older than 8 years who weigh less than 25 kg: inject half of the medicine (0.5 ml) - this is marked as "0.5” on the syringe.
• Children older than 8 years or children younger than 8 years who weigh more than
25 kg: inject all of the medicine (1 ml) - this is marked as " 1 ” on the syringe.
If you are given more GlucaGen® than you should
Too much GlucaGen® may lead to nausea and cause you to be sick (vomit). Specific treatment is not usually necessary.
Driving and using machines
Wait until the effects of very low blood sugar have worn off, before driving or using any tools or machines.
GlucaGen® HypoKit contains latex
The cap of the syringe contains latex rubber. This may cause severe allergic reactions in people who are allergic to latex.
3. How to use GlucaGen® HypoKit
Always use this medicine exactly as described in this leaflet or as your doctor has told you.
Check with your doctor or pharmacist if you are not sure.
Preparing and giving the injection
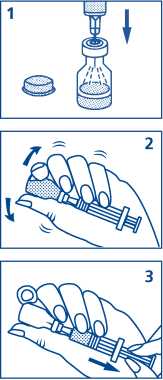
1. Remove the plastic cap from the vial. Pull the needle cover off the syringe. Do not remove the plastic back-stop from the syringe. Insert the needle through the rubber stopper (within the marked circle) of the vial containing GlucaGen® and inject all the liquid from the syringe into the vial.
2. Without taking the needle out of the vial, gently shake the vial until GlucaGen® has completely dissolved, and the solution is clear.
3. Make sure the plunger is completely down. While keeping the needle in the liquid, slowly withdraw all the solution back into the syringe. Do not pull the plunger out of the syringe. It is important to remove any air bubbles from the syringe:
• With the needle pointing upwards, tap the syringe with your finger
• Push the plunger slightly to release any air that has collected at the top of the syringe.
Continue to push the plunger until you have the correct dose for injection. A small amount of liquid will be pushed out when you do this. See How much to use, below.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects may happen with this medicine:
Tell your doctor immediately if you notice any of the following serious side effects: Very rare: may affect up to 1 in 10,000 people
• allergic reaction - the signs may include wheezing, sweating, rapid heart beat, rash, swollen face and collapse.
► Talk to a doctor immediately, if you notice any of the above serious side effects.
Other side effects
Common: may affect up to 1 in 10 people
• feeling sick (nausea).
Uncommon: may affect up to 1 in 100 people
• being sick (vomiting).
Rare: may affect up to 1 in 1,000 people
• stomach (abdominal) pain.
► If you get any side effects above, talk to your doctor. This includes any possible side effects not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects, you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard
5. How to store GlucaGen® HypoKit
• Keep this medicine out of the sight and reach of children
• Store either:
- in a refrigerator (2°C to 8°C), or
- out of a refrigerator below 25°C for up to 18 months within the shelf life period.
• Store in the original package to protect it from light
• Do not freeze, to prevent damage to the product
• Use immediately after mixing - do not store for later use
• Do not use this medicine after the expiry date - this is on the label. The expiry date refers to
CM
O
O
O
CNl
o
'=3-
OO
8-9402-01-010-2_v1-11:Layout 1
2015-05-28
4:56 PM
Page
2

the last date of that month.
• Do not use if the mixed solution looks like a gel or if any of the powder has not dissolved properly.
• Do not use if the plastic cap is loose or missing when you receive the product - return the product to your local pharmacy
• Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What GlucaGen® contains
• The active substance is glucagon 1 mg as hydrochloride, produced in yeast by recombinant DNA.
• The other ingredients are lactose monohydrate, water for injections, hydrochloric acid and/or sodium hydroxide (for pH adjustment).
What GlucaGen® looks like and contents of the pack
GlucaGen® is supplied as a sterile white powder of glucagon in a vial, with a solvent in a disposable syringe. The powder is compacted. Once mixed, the solution contains glucagon 1 mg/ml.
Marketing authorisation holder and manufacturer
Novo Nordisk A/S Novo Alle
DK-2880 Bagsrard, Denmark
7. Additional information for medical professionals
Medical professionals should refer to all sections above before reading this additional information.
Due to the instability of GlucaGen® in solution, the product should be given immediately after reconstitution and must not be given as an intravenous infusion.
Do not attempt to put the cap back on the needle of the used syringe. Place the used syringe in the orange box and dispose the used needle in a sharps container at the next available opportunity.
Treatment of severe hypoglycaemia
Administer by subcutaneous or intramuscular injection. If the patient does not respond within 10 minutes, intravenous glucose should be given. When the patient has responded to the treatment, give oral carbohydrate to restore the liver glycogen and prevent relapse of hypoglycaemia.
Diagnostic procedures
Oral carbohydrates should be given when the procedure has ended, if this is compatible with the diagnostic procedure concerned. Remember that GlucaGen® has the opposite effect of insulin. With endoscopy or radiography procedures, take extra care in administering GlucaGen® to patients with diabetes or to elderly people with a heart condition.
Note that a syringe with a thinner needle and a finer graduation may be more suitable for use in diagnostic procedures.
Examination of the gastrointestinal tract:
Doses vary from 0.2 - 2 mg depending on the diagnostic technique used and the route of administration. The diagnostic dose for relaxation of the stomach, duodenal bulb, duodenum and small bowel is 0.2 - 0.5 mg given intravenously or 1 mg given intramuscularly. The dose to relax the colon is 0.5 - 0.75 mg intravenously or 1 - 2 mg intramuscularly. The onset of effect after an intravenous injection of 0.2 - 0.5 mg occurs within one minute and the duration of the effect is between 5-20 minutes. The onset of action after an intramuscular injection of 1 - 2 mg occurs after 5-15 minutes and lasts approximately 10-40 minutes.
Additional side effects after use in diagnostic procedures
Blood pressure changes, rapid heartbeat, hypoglycaemia and hypoglycaemic coma.
GlucaGen® is a trademark owned by Novo Nordisk A/S, Denmark
© 2015
Novo Nordisk A/S
novo nordisk