Water For Injections
Out of date information, search another|
Brand name: GRANOCYTE |
Article: LEAFLET |
Date of creation: 30/06/2014 |
Tech. specification: MAI-P003863 |
Plant code: DM: 521150 |
|
Dosage: 13/34 MUI |
Item code: 521150 |
By: M. CASTRO |
Tech. plan: MAI-P003863-3b |
Country example: NA |
|
Quantity: 5 FL+SRG |
Current item code: 520679 |
Update: V1 |
Size (mm): 420x297 |
Font size: 9,5 pt mini |
|
Country: IRLANDE |
GMID Code: 560060 |
Date of modification: NA |
210x37,12 folded | |
|
Manuf. site: Maisons-Alfort |
By: NA | |||
|
Colours used: 2 ^ PANTONE BLACK ^ PANTONE 300 ^ PLAN ^ DECOUPE | ||||

PATIENT LEAFLET: INFORMATION FOR THE USER
GRANOCYTE 13 million IU/mL, powder and solvent for solution for injection/infusion in a pre-filled syringe
GRANOCYTE 34 million IU/mL, powder and solvent for solution for injection/infusion in a pre-filled syringe
Lenograstim
Read all of this leaflet carefully before you take this medicine.
• Keep this leaflet. You may need to read this again
• If you have any further questions ask your doctor or pharmacist
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours
• If any side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Granocyte is and what it is used for
2. Before you have Granocyte
3. How Granocyte is given
4. Possible side effects
5. How to store Granocyte
6. Further information
The name of your medicine is Granocyte, powder and solvent for injection/infusion (called Granocyte in this leaflet). Granocyte contains a medicine called lenograstim. This belongs to a group of medicines called cytokines.
Granocyte works by helping your body to make more of the blood cells which fight infection.
• These blood cells are made in your bone marrow.
• Granocyte encourages your bone marrow to make more cells called ‘blood stem cells’.
• It then helps turn these young blood cells into fully working blood cells.
• In particular, it helps produce more white blood cells called neutrophils. Neutrophils are important in fighting infections.
Granocyte is used:
• After cancer treatment, if the level of your white blood cells is too low (called ‘neutropenia’)
Some treatments for cancer (also called ‘chemotherapy’) affect the bone marrow. This can lower the number of your white cells. It particularly affects the neutrophils and is called ‘neutropenia’.
It lasts until your body is able to produce more white blood cells. When you have a low number of neutrophils it is easier to get infections. These can sometimes be very serious. Granocyte will help reduce the time you have low levels of cells. It does this by encouraging your body to make new white blood cells.
• When you need to increase your own blood stem cells (called ‘mobilisation’)
Granocyte can be used to encourage your bone marrow to produce blood stem cells. This is called ‘mobilisation’. This can happen alone or possibly after chemotherapy. These blood stem cells are taken out of your blood and collected using a special machine. The blood stem cells can then be stored and given back to you in a transfusion.
• After a bone marrow or blood stem cell transplant
When you are going to have a bone marrow or a blood stem cell transplant, you are first given a high dose chemotherapy or total body irradiation. This is to kill your sick cells. A bone marrow or blood stem cell transplant is then given to you as a blood transfusion. It will take some time for your new bone marrow to start producing new blood cells (including the white blood cells). Granocyte will help your body to speed up the recovery of your new white blood cells.
• When you want to donate your blood stem cells
Granocyte can also be used in healthy donors. Here they encourage the bone marrow to produce extra blood stem cells. This is called mobilisation - see above. These healthy donors can then donate blood stem cells to someone who needs them.
Granocyte can be used in adults, adolescents, and children older than 2 years.
Do not have this medicine and tell your doctor if:
• You are allergic (hypersensitive) to lenograstim or any of the other ingredients of Granocyte (listed in Section 6 below). Signs of an allergic reaction include: a rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue
• You have something called ‘phenylketonuria’
x-
The following information is intended for medical or healthcare professionals only:
Practical information on preparation and handling of the medicinal product for medical or healthcare professionals
Granocyte vials are for single-dose use only.
In view of the possible risk of microbial contamination, pre-filled syringe with solvent are for single use only.
Granocyte is for sub-cutaneous or intravenous use.
Preparation of the reconstituted solution
Aseptically add the extractable contents of one pre-filled syringe to the Granocyte vial using the 19G needle.
• Agitate gently until completely dissolved.
• Do not shake vigorously.
• The reconstituted parenteral solution appears transparent and free of particles.
• Withdraw the required volume of the reconstituted solution from the vial, using the 19G needle.
• Administer immediately by sub-cutaneous injection using the 26G needle.
In case of intravenous use Granocyte has to be diluted after reconstitution.
Granocyte is compatible with the commonly used administration sets for injection when diluted:
• in a 0.9% saline solution (polyvinyl chloride bags and glass bottles)
• or in a 5% dextrose solution (glass bottles)
Dilution of GRANOCYTE 13 million IU/mL to a final concentration of less than 0.26 million International Units/mL (2 ^g/mL) is not recommended. 1 vial of reconstituted GRANOCYTE 13 million IU/mL should not be diluted in more than 50 mL under any circumstances
Dilution of GRANOCYTE 34 million IU/mL to a final concentration of less than 0.32 million International Units/mL (2.5 ^g/mL) is not recommended. 1 vial of reconstituted GRANOCYTE 34 million IU/mL should not be diluted in more than 100 mL under any circumstances.
Any unused product/solution or waste material should be disposed of in accordance with local requirements ™Granocyte is a trademark of Chugai Pharma UK Limited.
©The text of this leaflet is copyright Chugai Pharma UK Limited.
• You have a type of cancer called ‘myeloid cancer’. However, you can have Granocyte if you have newly diagnosed ‘acute myeloid leukemia’ in certain cases, if you are more than 55 years old.
• You are having cancer chemotherapy on the same day.
Do not have this medicine if any of the above apply to you. If you are not sure talk to your doctor or pharmacist before being given Granocyte.
Take special care with Granocyte
Check with your doctor or pharmacist before having this medicine if:
• You have ever had any illness, especially allergies, infections, kidney or liver problems.
If you are not sure if this applies to you, talk to your doctor or pharmacist before having Granocyte.
Children and adolescents
Check with your doctor before having this medicine if:
• You have a type of cancer called ‘acute lymphocytic leukaemia’ and if you are less than 18 years old.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines.
This includes medicines obtained without a prescription, including herbal medicines.
If you want to donate your blood stem cells and you are having anti-coagulant treatment (such as warfarin or heparin), make sure the doctor knows this before starting with the Granocyte. Also tell them if you know you have any other blood clotting problems.
If you are given anti-cancer chemotherapy, do not use Granocyte during the period from 24 hours before the therapy starts to 24 hours after the therapy ends.
Pregnancy and breast-feeding
Granocyte has not been tested in pregnant women or in breast-feeding. Do not have this medicine if you are pregnant, might become pregnant or are breast-feeding unless your doctor tells you it is necessary. Ask your doctor or pharmacist for advice before taking any medicine if you might be pregnant.
Driving and using machines
The effect of Granocyte on the ability to drive or use machine or tools is not known. Wait to see how Granocyte affects you before driving or using tools or machines.
Important information about some of the ingredients of Granocyte
Granocyte contains phenylalanine. This may be harmful to you if you have something called ‘phenylketonuria’.
The tip cap composition of the pre-filled syringe contains latex rubber which may cause severe allergic reactions in predisposed subjects. (See ‘Do not take’ section above).
Granocyte must be given under supervision at an experienced Oncology or Haematology centre. It will normally be given by a doctor, nurse or pharmacist. It is given in an injection or an infusion. However, some patients have been taught how to give themselves the injection. If you have any questions about how this medicine is given, speak to your doctor, nurse or pharmacist.
How much Granocyte is given
If you are not sure why you are being given Granocyte or have any questions about how much Granocyte is being given to you, speak to your doctor, nurse or pharmacist.
After a bone marrow transplant, chemotherapy or for mobilisation of blood stem cells after chemotherapy
• Your doctor will work out how much to give you, depending on the surface area of your body. This is worked out using your weight and height. It is measured in ‘square metres’, which is written as ‘m2’
• The usual dose of Granocyte is 150 micrograms for each m2 of body surface area each day. The dose in children older than 2 years and adolescent is the same as in adults.
• The number of days you get Granocyte will be decided by your doctor. You may be given it for up to 28 days
• When Granocyte is given for mobilisation of blood stem cells after chemotherapy, your doctor will tell you when the collection of your blood stem cells will happen
For blood stem cell mobilization with Granocyte alone
• Your doctor will work out how much to give you, depending on your weight.
• The usual dose of Granocyte is 10 micrograms for each kg body weight each day. The dose in children older than 2 years and adolescent is the same as in adults.
• You will be given Granocyte as an injection beneath the skin for 4 to 6 days
• Collection of your blood stem cells will happen between 5 and 7 days later.
GRANOCYTE 13 million IU/mL can be used in patients with body surface area up to 0.7 m2. GRANOCYTE 34 million IU/mL can be used in patients with body surface area up to 1.8 m2.
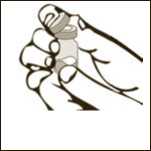
Diagram 1
Remove the plastic cap from the vial.
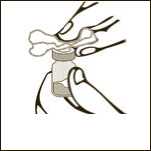
Diagram 2
Clean the rubber stopper.

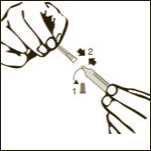
Diagram 3
Remove one pre-filled syringe from the blister and the two needles (one with the beige cone (19G) and one with the brown cone (26G).
<N
|
Brand name: GRANOCYTE |
Article: LEAFLET |
Date of creation: 30/06/2014 |
Tech. specification: MAI-P003863 |
Plant code: DM: 521150 |
|
Dosage: 13/34 MUI |
Item code: 521150 |
By: M. CASTRO |
Tech. plan: MAI-P003863-3b |
Country example: NA |
|
Quantity: 5 FL+SRG |
Current item code: 520679 |
Update: V1 |
Size (mm): 420x297 |
Font size: 9,5 pt mini |
|
Country: IRLANDE |
GMID Code: 560060 |
Date of modification: NA |
210x37,12 folded | |
|
Manuf. site: Maisons-Alfort |
By: NA | |||
|
Colours used: 2 ^ PANTONE BLACK ^ PANTONE 300 ^ PLAN ^ DECOUPE | ||||
If you have more Granocyte than you should
If you are given this medicine by a doctor, nurse or pharmacist it is unlikely they will give you too much medicine. They will monitor your progress and check the dose. Always ask if you are not sure why you are getting a dose of medicine.
If you give yourself too much Granocyte, tell a doctor or go to the hospital straight away. Take this medicine pack with you. This is so the doctor knows what you have taken. You may get particularly bad side effects if you have too much. The most likely problem you may have is muscle and bone pain.
If you miss a dose of Granocyte
Do not have a double dose to make up for a forgotten injection. Always speak to your doctor who will tell you what you should do.
Blood tests
A doctor needs to monitor you while you are having this medicine. They will need to do regular blood tests. These will check the level of your different blood cells (neutrophils, other white blood cells, red blood cells, platelets).
Other blood tests which may be taken by other doctors can show changes while you are having Granocyte. If you are having blood tests, it is important to tell the doctor that you are having Granocyte. The number of your white blood cells may go up, the number of your platelets may go down and there may be an increase in enzyme levels. These changes usually improve after you have stopped having Granocyte. If you are having blood tests, it is important to tell the doctor you are having Granocyte.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, Granocyte can cause side effects, although not everybody gets them.
Stop having Granocyte and tell your doctor straight away if:
• You get a pain in your upper left side of your tummy or your left shoulder. These could be signs of an increase in the size of your spleen. This is a common side effect but a very rare side effect may cause the spleen to split.
• You have an allergic reaction. The signs include a rash, swallowing and breathing problems,
• swelling of your lips, face, throat or tongue. This is a very rare side effect.
• You have a very serious allergic reaction called ‘anaphylactic shock’. The signs include feeling faint, weakness, difficulty breathing or swelling of the face. This is a very rare side effect.
• You have breathing problems. The signs include cough, fever or you may easily become out of breath. This is a rare side effect
Tell a doctor or pharmacist as soon as possible if you have any of the following side effects:
• A reaction at the site of the injection. This is a common side-effect.
• Skin problems like plum coloured, raised areas on your arms or legs and sometimes your face or neck with fever (signs of ‘Sweet’s syndrome’). There may also be raised red lumps with fever and headache (signs of ‘Lyell’s syndrome’). Also, other skin problems like red raised bruises on the legs or ulcers on your body with fever and joint pain. These are very rare side effects.
Other side effects include:
• Pains in your bones and muscles and a headache. This is a common side effect. If it happens the pain can be controlled with normal painkillers.
Blood stem cell donors
Like any medicine, Granocyte can cause side effects, although most people do not get them. Some of
the side-effects may occur immediately while others may take a few days to occur.
Tell your doctor immediately if:
• You get pain in the upper left side of your tummy or your left shoulder. These could be signs of an increase in the size of your spleen, which is a common side effect called splenomegaly. This could lead to your spleen splitting, which is a very rare side effect.
• You have signs of an allergic reaction, even after the first administration of Granocyte. The signs include a rash, problems swallowing or breathing, and swelling of your lips, face, throat or tongue. This is a very rare side effect.
• You have a very rare and very serious allergic reaction called ‘anaphylactic shock’. This is a sudden life-threatening reaction. The signs include feeling faint, weakness, difficulty breathing or swelling of the face.
• You experience cough, fever and difficulty breathing (dyspnoea). These can be signs of Acute Respiratory Distress Syndrome (ARDS) which is a very rare side effect.
• You have any of the following or combination of the following side effects: swelling or puffiness, which may be associated with passing water less frequently, difficulty breathing, abdominal swelling and feeling of fullness, and a general feeling of tiredness. These symptoms generally develop in a rapid fashion. These could be symptoms of an uncommon (may affect up to 1 in 100 people) condition called “Capillary Leak Syndrome” which causes blood to leak from the small blood vessels into your body and needs urgent medical attention.
-x----------------
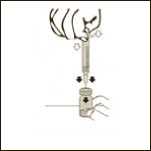
Diagram 5
Keeping the vial on a flat surface, push the needle trough the rubber stopper and push the plunger rod to inject the solvent into the vial
Tell a doctor if you have any of the following very common side effects:
• You may have pain, pain in your bones and back, headache, fever, and/or feel sick (nausea).
• You may get temporary alterations in blood tests including those related to your liver function, which usually do not require any additional precautions and normalize after the drug is stopped.
• After the donation of the blood stem cell you may feel tired. It is because of the fall in your red blood cells. You may also have a decrease in platelets count which could make you bleeding or bruising more easily than normal.
All the above effects will return to normal in the days following the donation.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly (see details below).
UK - Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard
Ireland - HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie. By reporting side effects you can help provide more information on the safety of this medicine.
Keep out of the reach and sight of children.
Do not use any of the parts of the Granocyte powder and solvent for solution kit after the expiry (EXP) date. The expiry date for Granocyte powder is given on the outer carton box and on the label of each vial of Granocyte. The expiry date for the solvent (water for injection) is given either on the label of each ampoule of water for injection, or both on the label of the water pre-filled syringe and on the paper foil of the blister.
The expiry date refers to the last day of that month.
Do not store above 30°C. Do not freeze.
After reconstitution or dilution immediate use is recommended. If needed, you may store the reconstituted or diluted solution up to 24 hours at 2°C -8°C (in a refrigerator).
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
What Granocyte contains
• The substance active is lenograstim (rHuG-CSF) 13.4 million International Units (equivalent to 105 micrograms) per mL after reconstitution.
• The substance active is lenograstim (rHuG-CSF) 33.6 million International Units (equivalent to 263 micrograms) per mL after reconstitution .
• The other ingredients contained in the powder are arginine, phenylalanine, methionine, mannitol (E421), polysorbate 20 and diluted hydrochloric acid.
• Excipients known to have a recognised action or effect: phenylalanine
• The solvent used to reconstitute the solution is Water for Injections What Granocyte looks like and contents of the pack(s)
Granocyte is presented as [powder and a solvent for solution for injection/infusion in a pre-filled syringe].
Powder in a vial + 1mL of solvent in a pre-filled syringe with two needles (the larger white one for reconstitution (19G) and the smaller brown one for administration (26G))
Granocyte is presented as [powder and a solvent for solution for injection/infusion].
Powder in a vial + 1mL of solvent in ampoule (UK market only).
GRANOCYTE is available in pack sizes of 1 or 5.
Not all pack sizes may be marketed Marketing Authorisation Holder
Chugai Pharma UK Ltd, Mulliner House, Flanders Road, Turnham Green, London W4 1NN. Manufacturer
SANOFI WINTHROP INDUSTRIE, Usine de Maisons-Alfort, 180 rue Jean-Jaures, BP40,
94702 Maisons-Alfort - Cedex - France
This medicinal product is authorised in the Member States of the EEA under the following names:
All Member States of the EEA: GRANOCYTE Italy: GRANOCYTE and MYELOSTIM
For any information about this medicine, please contact the local representative of the Marketing Authorisation Holder:
Chugai Pharma UK Ltd, Mulliner House, Flanders Road, Turnham Green, London W4 1NN. This leaflet was last approved in July 2014

CHUGAI
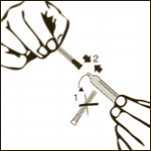

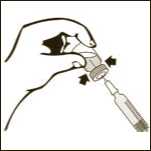

Diagram 8
Pull back slowly the plunger rod and withdraw the prescribed dose. Withdraw the required volume from the vial.
Diagram 7
Keeping the needle and the syringe attached to the vial, turn the vial upside down. Make sure the needle tip is in the solution
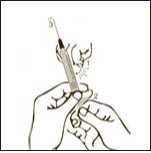
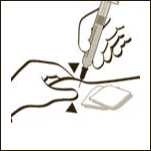
Diagram 11
If necessary adjust the volume to be administered. GRANOCYTE is now ready for administration. Administer immediately by sub-cutaneous injection.
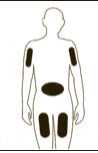
Diagram 10
Remove any air bubbles by gently tapping on the body of the syringe and push slowly the plunger rod to eliminate the air
o
c
c
sc
<0
<N
<0