Salamol Cfc-Free Inhaler 100 Micrograms Pressurised Inhalation Suspension

Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor, pharmacist or nurse. This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
PE3274

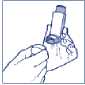
3. Breathe out normally as far as you comfortably can. Then hold the mouthpiece of the inhaler firmly between your lips.
What is in this leaflet
1. What Salamol is and what it is used for
2. What you need to know before you use Salamol
3. How to use Salamol
4. Possible side effects
5. How to store Salamol
6. Contents of the pack and other information
1. What Salamol is and what it is used for
Effect
Salamol contains salbutamol sulfate, which belongs to a group of medicines called beta agonists. Salbutamol is a bronchodilator and works by widening the airways in your lungs to allow air in and out. This helps you to feel less breathless, wheezy or tight-chested.
Usage
Salamol is used to treat asthma in adults, adolescents and children aged 4 to 11 years. It can also be used to prevent asthma caused by exercise or asthma caused by a reaction to allergens (substances to which you are allergic to e.g. house dust, pollen, dog hair, cat hair, cigarette smoke, etc.) or to treat breathing difficulties associated with reversible airways obstruction (e.g. chronic obstructive pulmonary disease [COPD]).
This type of medicine is known as a ‘reliever'. You may be using another medicine to prevent you from having an asthma attack (“preventer"). You can safely use your preventer with this medicine.
2. What you need to know before you use Salamol
Do not use Salamol
- If you are allergic to salbutamol or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Salamol
- if you suffer from thyroid problems, diabetes, serious heart disease, fast irregular heart rhythms or high blood pressure.
- if you have a condition known as hypoxia
(a disorder where the oxygen level in the blood is low and may cause breathlessness) or if you have a history of heart disease or angina.
Consult a doctor immediately if your usual treatment is not working or you need more than 8 puffs per day (for adults) or 4 puffs per day (for children) or in case of worsening asthma symptoms. Your dosage should only be increased on medical advice.
Other medicines and Salamol
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
If you are having treatment that requires a general anaesthetic, please tell your anaesthetist that you are taking Salamol. Do not use this medicine for at least six hours before the intended anaesthetic. Salbutamol can reduce the amount of potassium in your blood. If this happens, you may notice an irregular heartbeat, muscle weakness or cramp. This is more likely to happen if you use salbutamol with some medicines used to treat high blood pressure, other medicines used to treat breathing problems (e.g. steroids), stimulants (e.g. xanthines) and long-term laxatives.
The following medicines may influence the effect of Salamol:
• Some medicines for the treatment of high blood pressure such as beta-blockers (e.g. propranolol);
• Medicines used to treat heart disease (e.g. digoxin);
• Medicines for the treatment of depression (from the monoamine oxidase inhibitor group e.g. phenelzine or tricyclic antidepressants e.g. amitriptyline or trazodone);
• Water tablets (diuretics);
• Medicines for the treatment of chronic alcoholism (e.g. disulfiram);
• The anti-microbial drug metronidazole.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Salamol does not affect your ability to drive or to
use machinery.
3. How to use Salamol
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The dosage is individually adjusted according to your doctor's instructions, based on previous asthma therapy.
For the best results with this medicine you should use it when required to relieve symptoms of asthma and other chest conditions with similar symptoms, such as wheezing, breathlessness and tightness in your chest. Take one puff as a starting dose. This may be increased to two puffs if necessary.
To prevent asthma caused by exercise or allergens (e.g. house dust, pollen etc), take two puffs 10-15 minutes before you exercise or before exposure to known allergens.
Do not take more than eight puffs in 24 hours.
Wait 4 hours between doses.
Use in children and adolescents
The usual dose for children under the age of 12 years to relieve asthma symptoms such as wheezing, breathlessness and tightness in the chest, is one puff as a starting dose. This may be increased to two puffs if necessary.
Children over the age of 12 should refer to the adult dosage instructions above.
The usual dose for children under the age of 12 years to prevent asthma caused by exercise or allergens (e.g. house dust, pollen etc), is one puff 10-15 minutes before exercise or before exposure to known allergens.
This can be increased to two puffs if necessary. Children over the age of 12 should refer to the adult dosage instructions above.
The usual dose for children under the age of 12 years for regular treatment of asthma is up to 2 puffs, 4 times a day.
Do not take more than eight puffs in 24 hours. Wait 4 hours between doses.
Instructions for use Important
Before using this medicine, please read this leaflet carefully and follow the instructions.
An adult should always supervise children when they use Salamol. Children may need help to use their inhaler. Parents can help by spraying the aerosol when the child begins to breathe in.
Always remember the following:
• Wait four hours between doses.
• Tell your doctor if your asthma gets worse or if the inhaler does not provide as much relief from your asthma as before.
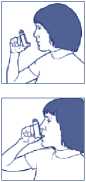
• You should sit or stand upright while taking this medicine. It is important when using this medicine to hold the inhaler upright
as it will not work if it is not held in this position.
Test spray the inhaler by firing two shots into the air before you use it for the first time and also if you have not used it for a period of five days or more.
Using the Inhaler
If you or your child find it difficult to use the inhaler, your doctor or healthcare provider may be recommend using the Volumatic® spacer device with the inhaler. Your doctor, nurse, pharmacist, or other healthcare provider should show you how to use the Volumatic® spacer with your inhaler and show you how to care for your Volumatic spacer, and will answer any questions that you may have. It is important that if you are using the Volumatic spacer with your inhaler that you do not stop using it without talking to your doctor or nurse first. If you stop using a spacer device your doctor may need to change the dose of medicine required to control your asthma. Always talk to your doctor before making any changes to your asthma treatment.
If you are not using a Volumatic spacer, ignore the steps specifically marked for Patients using a Volumatic spacer and continue to the next step.
If you are using a Volumatic spacer follow all the steps below including the ones for Patients using a Volumatic spacer.
1. Take the cap off the inhaler. Make sure the mouthpiece is clean and clear of fluff and dirt.
Patients using a Volumatic spacer - Fit the
two halves of the Volumatic device together. Line up the notch on one half with the slot on the other (these are marked with small arrows), then press the two halves firmly together.
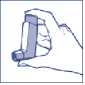
2. Hold the inhaler upright, with your thumb on the base and your first finger on the top of the canister*. Now shake the inhaler vigorously up and down.
Patients using a Volumatic spacer - Fit the
inhaler into the end of the Volumatic, opposite the mouthpiece. Check the mouthpiece of the Volumatic inside and outside to make sure that it is clean
4. Breathe in slowly and deeply. At the same time as you start to breathe in, press the aerosol canister with your first finger to spray the aerosol and release the medicine.
Continue to breathe in slowly and deeply.
Patients using a Volumatic spacer - Press down with your finger(s) on the top of the inhaler to release one puff of medicine. Straight away take one deep steady breath or 5 normal breaths to make sure that the medicine goes into your lungs.
You do not need to take the Volumatic out of your mouth to breathe out. You should hear the mouthpiece valve ‘click' or rattle as you breathe through it. If you do not hear it, tilt the Volumatic up slightly and try again.
5. Take the inhaler out of your mouth and hold your breath for 10 seconds, or for as long as you comfortably can. Breathe out slowly.
Patients using a Volumatic spacer - Take the Volumatic spacer out of your mouth and take your finger from the top of the inhaler. Continue holding your breath for a few seconds, or as long as is comfortable. Breathe out slowly.
6. If you need more than one puff, wait about one minute and then start again from step 2. Put the cap back on the inhaler.
7. You must keep your inhaler clean, especially in the mouthpiece. This will prevent deposits from the aerosol building up. To prevent your inhaler becoming blocked you must clean it once a week.
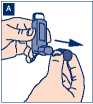
B Remove the metal canister from the plastic mouthpiece. Do not put the metal canister into water.
C Rinse the inhaler mouthpiece and the mouthpiece cap with warm running water for at least 30 seconds.
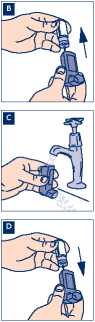
Failure to allow the mouthpiece to dry properly will result in an increase in blockage problems,
8. What to do if your inhaler stops working properly? It is important to clean your inhaler regularly to prevent deposits from the aerosol building up and blocking the inhaler. If your inhaler does not seem to be working, it may be that your inhaler has become blocked and you should clean your inhaler according to the section "Cleaning the inhaler".
If your inhaler still does not work, return it to your doctor or pharmacist.
Important: Do not rush steps 3 and 4. It is important that you start to breathe in as slowly as possible just before using your inhaler. Practice in front of the mirror for the first few times. If you see "mist" coming from the top of the inhaler or the sides of your mouth you should start again from step 2. *Note: Children and people with weak hands may find it easier to hold the inhaler with both hands, in this case for step 2 put your two first fingers on the top of the canister and both thumbs on the base of the inhaler.
■/This product can be used with theVolumatic® Spacer. Please refer to the instruction leaflet for the Volumatic spacer device for further information.
Cleaning the inhaler
Keeping the plastic mouthpiece clean is very important as it will help prevent your inhaler becoming blocked. You must clean your inhaler once a week.
A To clean your inhaler first remove the mouthpiece cap.
D Shake off any excess water and dry the plastic mouthpiece and mouthpiece cap thoroughly (leave to dry overnight if possible) but do not use direct heat. Put the metal canister back in your inhaler. Replace the cap.
If you need to use your inhaler before it is dry, shake off ary water from the plastic mouthpiece and put the canister back in. Test spray the inhaler by firing two puffs in the air before taking your usual dose. Wash and dry the mouthpiece again as described above. Cleaning theVolumatic spacer
YourVolumatic should be cleaned regularly, preferably twice a week.
A Gently pull the two halves of theVolumatic apart. Do not take the valve apart.
B Wash the two halves of theVolumatic in warm water, which can contain a mild detergent or a sterilising solution of the type used to clean babies' bottles. You can use a soft toothbrush or bottle to help remove any film which forms on the valve or the i nside of the Volumatic. As long as the valve still moves when you breathe in and out, this deposit will not affect the working of theVolumatic.
C Rinse thoroughly with clean water. Leave the parts at room temperature until they are completely dry. Do not rub the inside of your Volumiatic with a cloth or polish as this miay cause static electricity which can affect the miedicine. Do not put the Volumiatic in a heated place to dry miore quickly.
D Store the Volumiatic in the carton to keep clean.
Children can assembly the Volumiatic and clean it as necessary under adult supervision.
The Volumiatic miay need to be replaced after about 6 to 12 mionths of use.
If you use more Salamol than you should It is important that you take your dose as stated on the pharmacist's label or as advised by your doctor. You should not increase or decrease your dose without seeking medical advice.
If you accidentally take a larger dose than recommended, you miay notice that your heart is beating faster than usual, that you feel shaky or tense, you miay have a headache and your skin miay look flushed and feel hot. These effects usually wear off in a few hours, but you should tell your doctor as soon as possible. Your doctor miay want to check your blood potassium levels.
4. Possible side effects
Like all miedicines, this miedicine can cause side effects, although not everybody gets themi.
Contact your doctor immediately if you experience allergic (hypersensitivity) reactions.These include
• Wheezing, coughing and difficulty breathing;
• Rash, swelling of the face and throat and a fall in blood pressure.
You might collapse in very rare circumstances.
If you experience any of these side effects or if they occur suddenly after using salbutarriol you should stop using your medication straightaway and tell your doctor immediately. Allergic reactions to salbutarriol are very rare (occurring in less than 1 in 10,000 patients).
Other side effects:
Common (may affect up to 1 in 10 people):
• Feeling tense and getting headaches (which are more likely with higher doses);
• Feeling shaky (muscle tremors);
• Dizziness.
Uncommon (may affect up to 1 in 100 people):
• Muscle pain (myalgia).
Rare (may affect up to 1 in 1,000 people):
• Reduction in the amount of potassium in your blood (if this happens, you may notice an irregular heartbeat, muscle weakness or cramp);
• Increase in lactate levels and acid levels in the blood (you may experience symptoms such as persistent nausea and vomiting, unexplained tiredness, shortness of breath and rapid breathing, cold or blue hands and feet);
• Sleep disturbances and sensing things that are not real;
• Rapid or irregular heart beat (tachycardia or palpitations);
• Increased blood flow to your extremities (peripheral dilatation)
• Mouth and throat irritations, nausea, vomiting and dry and sore mouth;
• Muscle cramps.
Very rare (may affect up to 1 in 10,000 people):
• Difficulty in sleeping (insomnia);
• Itching of your skin;
• Trembling (particularly your hands).
Not known (frequency cannot be estimated from the available data):
• Chest pain (due to heart problems such as angina).
Additional side effects in children
• Sleep disturbances and sensing things that are not real have been reported.
• Flyperactivity.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist, but do not stop using this medicine unless you are told to do so.
Reporting of side effects
If you get any side effects, talk to your doctor,
pharmacist or nurse.This includes any possible side
effects not listed in this leaflet. You can also report
side effects directly via the Yellow Card Scheme at:
By reporting side effects you can help provide more
information on the safety of this medicine.
5. How to store Salamol
Keep this medicine out of the sight and reach of children.
Do not store above 25°C.
Do not refrigerate or freeze. If this medicine gets very cold, remove the metal canister from the inhaler and warm it in your hands for a few minutes before you use it. Do not warm the canister in any other way.
Pressurised aerosol canister. Do not puncture, break or bum the canister, even if it seems empty. Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date refers to the last day of that month.
Do not throw away medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Salamol contains
- The active substance is salbutamol sulfate equivalent to 100 micrograms of salbutamol in each puff (metered dose).
- The other ingredients are ethanol anhydrous (alcohol) and the propellant norflurane (FlFA-134a). This medicine does not contain any CFCs.
What Salamol CFC-Free Inhaler lookls like and contents of the pack
• The name of your medicine is Salamol CFC-Free Inhaler 100 micrograms Pressurised Inhalation Suspension.
• Each pack of Salamol contains a single inhaler and canister which supplies 200 metered doses.
MarketingAuthorisation Holder:
Norton Flealthcare Ltd.,
T/A IVAX Pharmaceuticals UK,
Ridings Point,
Whistler Drive,
Castleford,
West Yorkshire,
WF10 5HX,
United Kingdom Manufacturer:
IVAX Pharmaceuticals Ireland IDA Industrial Park Waterford Ireland
This medicinal product is authorised in the Member States of the EEA under the following names:
Germany: Salbutamol Teva 100 Mikrograrrirri Dosieraerosol Druckgasinhalation, Suspension Luxembourg: ECOSAL 100 meg suspension pour inhalation en flacon pressurise Belgium: ECOSAL 100 pg aerosol, suspensie
This leaflet was last revised in 03/2016
01069-FLEA
Salamol CFC-Free Inhaler IVAX
100 micrograms Pressurised Inhalation Suspension
Salbutamol Sulfate