Salamol Cfc-Free Inhaler 100 Micrograms Pressurised Inhalation Suspension
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Salamol CFC-Free Inhaler
100 micrograms Pressurised Inhalation, suspension.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
One metered dose contains salbutamol sulfate equivalent to 100 micrograms salbutamol.
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Pressurised Inhalation, suspension
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Therapeutic Indications
The symptomatic treatment of asthma and other conditions with associated reversible airways obstruction. For relief of wheezing and shortness of breath, Salamol CFC-Free Inhaler should be used on an as required basis.
Prevention of asthma attacks induced by exercise or exposure to allergens.
Salamol CFC-Free Inhaler can be used as relief medication to manage mild, moderate and severe asthma, provided its use does not delay the introduction and regular use of inhaled corticosteroid therapy, where necessary.
Salamol CFC-Free Inhaler is indicated in adults, adolescents and children aged 4 to 11 years. For infants and children under 4 years of age, see section 5.1.
4.2 Posology and method of administration
Posology
For optimum results in most patients Salamol CFC-Free Inhaler should be used as required.
Adults (including the Elderly)
Relief of acute asthma symptoms including bronchospasm
One inhalation (100 micrograms) may be administered as a single minimum starting dose. This may be increased to two inhalations (200 micrograms) if necessary.
Prevention of allergen or exercise-induced bronchospasm
Two inhalations (200 micrograms) should be taken 10-15 minutes before challenge.
On demand use of Salamol CFC-Free Inhaler should not exceed 8 inhalations (800 micrograms) in any 24 hours. Inhalations should not usually be repeated more often than every 4 hours. Reliance on such frequent supplementary use, or a sudden increase in dose indicates poorly controlled or deteriorating asthma.
For all patients, four hours should be allowed between each dose.
Paediatric Population
Relief of acute asthma symptoms including bronchospasm
The usual dosage for children under the age of 12 years: one inhalation (100 micrograms). The dose may be increased to two inhalations (200 micrograms) if required.
Children aged 12 years and over: Dose as per adult population.
Prevention of allergen or exercise-induced bronchospasm
The usual dosage for children under the age of 12 years: one inhalation (100 micrograms) before challenge or exertion. The dose may be increased to two inhalations (200 micrograms) if required.
Children aged 12 years and over: Dose as per adult population.
The usual dosage for children under the age of 12 years: up to two inhalations (200 micrograms) 4 times daily.
Children aged 12 years and over: Dose as per adult population.
Patients with Hepatic or Renal Impairment No need to adjust the dose.
Method of Administration For Inhalation Use.
Salamol administration in children should be supervised by an adult. Children may need help to use their inhaler. Parents can help by spraying the aerosol when the child begins to inhale.
Patients should wait four hours between doses.
Patients should sit or stand upright during inhalation.
It is also important during inhalation that the inhaler be held in an upright position as the inhaler works correctly only in a vertical position.
The aerosol spray is inhaled through the mouth into the lungs. The inhaler should be tested by firing two shots into the air before first use, and if the inhaler has not been used for a period of five days or longer.
Use of the Inhaler
Salamol inhaler may be used with a Volumatic® spacer device by patients who find it difficult to synchronise aerosol actuation with inspiration of breath. Patients should refer to the instruction leaflet for the Volumatic spacer device for further information.
Patients not using a Volumatic spacer should be advised to ignore the steps specifically marked for Patients using a Volumatic spacer and continue to the next step.
Patients using a Volumatic spacer should be advised to follow all the steps below including the ones for Patients using a Volumatic spacer.
1. Patients should remove the cap from the inhaler mouthpiece and make sure the mouthpiece is clean and clear.
Patients using a Volumatic spacer should fit the two halves of the spacer together and then press them firmly together.
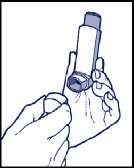
2. The inhaler must be held in a vertical position, with the thumb on the base and the index finger on the top of the canister*. Then, patients should shake the inhaler vigorously up and down.
Patients using a Volumatic spacer should fit the inhaler firmly into the end of the spacer, opposite the mouthpiece. Patients should be advised to make sure the mouthpiece is clean.
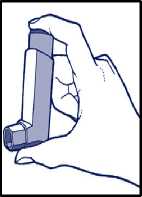
3. Patients should exhale normally, and place the mouthpiece in their mouth with their lips closed around it.

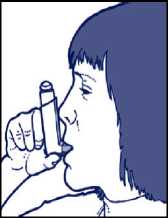
4. Patients should inhale slowly and deeply and press the canister at the same time to release the spray.
Patients using a Volumatic spacer should press down with their finger(s) on the top of the inhaler to release one puff of medicine. Then, they should take one deep steady breath or 5 normal breaths to make sure that the medicine goes into their lungs. Patients should be advised they do not need to take the Volumatic out of their mouth to exhale. They should hear the mouthpiece valve ‘click’ or rattle as they breathe through it. Otherwise, they should be advised to tilt the Volumatic up slightly and to try again.
5. Patients should remove the inhaler from their mouth and hold their breath for 10 seconds, and then exhale slowly.
Patients using a Volumatic spacer should remove it from their mouth and their finger(s) from the top of the inhaler, and continue to hold their breath for a few seconds, then exhale slowly.
6. If more than one puff is needed, it is important to advise the patient to wait about one minute and start again from step 2. Then, put the cap back on the inhaler.
Patient should be advised to clean the inhaler once a week ,especially in the mouthpiece to prevent deposits from the aerosol building up.
Patients should be advised not to rush Steps 3 and 4. It is important that the patient starts to breathe in as slowly as possible just before using the inhaler. If ‘mist’ is coming from the top of the inhaler or the sides of the mouth of the patient, they should be advised to start again from step 2.
*Note: Children and people with weak hands should be advised they may find it easier by holding the inhaler with both hands.
As with most inhaled medicinal products in pressurised containers, the therapeutic effect of this medicinal product may decrease when the container is cold.
The container should not be punctured, broken or burnt, even when apparently empty.
The metal container must not be put into water.
Full instructions for use of the inhaler and Volumatic spacer are given in the Patient Information Leaflet which should be read carefully by the patient before use.
Cleaning of the Inhaler
Patient must clean the inhaler once a week.
A Remove the mouthpiece cap
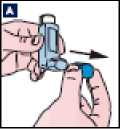
B Remove the canister from the plastic mouthpiece. Patient should be advised not to put the canister into water.
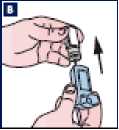
C Rinse the inhaler mouthpiece and mouthpiece cap with warm water for at least 30 seconds.
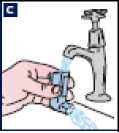
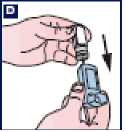
D Shake off any excess water and dry the plastic mouthpiece and mouthpiece cap thoroughly (overnight if possible). It is important to advise the patients not to use direct heat. Patients should put the canister back in the inhaler and replace the cap.
Cleaning of the spacer
The Volumatic should be cleaned regularly, preferably twice a week.
A Patients should gently pull the two halves of the spacer apart. They should be advised not to take the valve apart.
B The two halves of the Volumatic should be washed in warm water, which can contain a mild detergent or a sterilising solution of the type used to clean
babies’ bottles. Patient can use both toothbrush or bottle brush to help remove any film formed.
C Patients should rinse thoroughly with clean water and leave the parts at room temperature until they are completely dry. Patients should be advised not to rub the inside of the spacer with a cloth or polish as this may cause static electricity which can affect the medicine. Also, they should be advised not to introduce the spacer in a heated place to dry more quickly.
D The Volumatic should be stored in the cartoon to keep clean.
Children can clean their Volumatic when necessary under adult supervision.
Patients should be advised that they may need to replace the Volumatic after about 6 to 12 months of use.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1. Salbutamol inhalation is contraindicated in treatment of threatened abortion or premature labour.
4.4 Special warnings and precautions for use
Salbutamol should be administered cautiously to patients with thyrotoxicosis, coronary insufficiency, hypertrophic obstructive cardiomyopathy, arterial hypertension, known tachyarrhythmias, concomitant use of cardiac glycosides or diabetes mellitus.
Patients should be instructed in the proper use of the inhaler and their technique checked, to ensure that the active substance reaches the target areas within the lungs.
The management of asthma should normally follow a stepwise programme, and the patient’s response should be monitored clinically and by lung function tests. Increasing use of short-acting inhaled bronchodilators, in particular b2-agonists to control symptoms, indicates deterioration of asthma control. Under these conditions, the patient’s therapy plan should be reassessed.
Asthamatic patients whose conditions deteriorates despite salbutamol therapy, or where a previously effective dose fails to give relief for at least three hours, should seek medical advice in order that any necessary additional steps may be taken.
The dosage or frequency of administration should only be increased on medical advice.
Patients requiring long term management with Salamol CFC-Free Inhaler should be kept under regular surveillance.
Care should be taken when treating acute asthma attacks or exacerbation of severe asthma as increased serum lactate levels, and rarely, lactic acidosis have been reported after the use of high doses of salbutamol have been used in emergency situations this is reversible on reducing the dose of salbutamol.
Cardiovascular effects may be seen with sympathomimetic drugs, including salbutamol. There is some evidence from post-marketing data and published literature of rare occurrences of myocardial ischaemia associated with salbutamol. Patients with underlying severe heart disease (e.g. ischaemic heart disease, arrhythmias or severe heart failure) who are receiving salbutamol should be warned to seek medical advice if they experience chest pain or other symptoms of worsening heart disease. Attention should be paid to assessment of symptoms such as dyspnoea and chest pain, as they may be either respiratory or cardiac in origin.
Potentially serious hypokalaemia may result from B2-agonist therapy mainly from parenteral and nebulised administration. Particular caution is advised in acute severe asthma as this effect may be potentiated by concomitant treatment with xanthine derivatives, steroids, diuretics and by hypoxia. It is recommended that serum potassium levels are monitored in such situations.
In common with other beta-adrenoceptor agonists, salbutamol can induce reversible metabolic changes such as increased blood glucose levels. Diabetic patients may be unable to compensate for the increase in blood glucose and the development of ketoacidosis has been reported. Concurrent administration of glucocorticoids can exaggerate this effect.
As with other inhalation therapies, the potential for paradoxical bronchospasm should be considered. If it occurs the preparation should be discontinued immediately and alternative therapy given. Solutions which are not of neutral pH may rarely cause paradoxical bronchospasm in some patients. Salbutamol and non-selective beta blocking drugs such as propranolol should not usually be prescribed together.
4.5 Interaction with other medicinal products and other forms of interaction
Propranolol and other non-cardioselective B-adrenoreceptor blocking agents antagonise the effects of salbutamol, and should not usually be prescribed together.
Monoamine oxidase inhibitors, tricyclic antidepressants, digoxin: risk of increased cardiovascular effects.
Patients should be instructed to discontinue salbutamol at least 6 hours before an intended anaesthesia with halogenic anaesthetics, wherever possible.
Hypokalaemia occurring with ^2-agonist therapy may be exacerbated by treatment with xanthines, steroids, diuretics and long-term laxatives.
Because of the content of ethanol, there is theoretical potential for interaction in patients taking disulfiram or metronidazole
4.6 Fertility, pregnancy and lactation
Pregnancy
Salamol CFC-Free Inhaler should only be used in pregnancy in situations where the expected benefit to the mother is thought to outweigh any risk to the foetus.
Salbutamol inhalation is contraindicated in treatment of threatened abortion or premature labour.
Salamol CFC-Free Inhaler
There is no documented evidence of the use of salbutamol formulated with propellant HFA-134a in pregnant women.
Propellant HFA-134a
There is no documented evidence of the use of propellant HFA-134a in pregnant women. Pregnant animals exposed to high levels of HFA-134a showed no evidence of any adverse effects.
Salbutamol
Experience on the use of beta-sympathomimetics during early pregnancy indicates no harmful effect at the doses ordinarily used for inhalation therapy. High systemic doses at the end of pregnancy can cause inhibition of labour and may induce P2-specific foetal/neonatal effects like tachycardia and hypoglycaemia. Inhalation therapy at recommended doses is not expected to induce these harmful side effects at the end of pregnancy.
Breastfeeding
Salamol CFC-Free Inhaler should only be used in lactation in situations where the expected benefit to the mother is thought to outweigh any risk to the neonate.
Salamol CFC-Free Inhaler
There is no documented evidence of the use of salbutamol formulated with propellant HFA-134a in lactating women.
Propellant HFA-134a
There is no documented evidence of the use of propellant HFA-134a in lactating women. Lacting animals exposed to high levels of HFA-134a showed no evidence of any adverse effects.
Salbutamol
Salbutamol may be secreted in breast milk. It is not known whether salbutamol has a harmful effect on the neonate.
Fertility
There is no information on the effects of salbutamol on human fertility.
4.7. Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
4.8 Undesirable effects
Based on the MedDRA system organ class and frequencies, adverse events are listed in the table below.
Frequencies are defined as: very common (>1/10), common (>1/100 to <1/10), uncommon (>1/1000 to <1/100), rare (>1/10000 to <1/1000), very rare (<1/10000; including isolated reports), not known (cannot be estimated from the available data).
|
System Organ Class |
Frequency |
Adverse Event |
|
Immune system disorders |
Very rare |
Hypersensitivity reactions (angioedema, urticaria, bronchospasm, hypotension and collapse) |
|
Metabolism and nutrition disorders |
Rare |
Hypokalaemia ,increased serum lactate levels and acidosis lactic |
|
Psychiatric disorders |
Common Rare Very rare |
Tenseness Sleep disturbances and hallucinations (especially in children), hyperactivity in children Insomnia |
|
Nervous system disorders |
Common |
Tremor muscle, headache, dizziness |
|
Cardiac disorders |
Rare Very rare Not known |
Palpitations, tachycardia Cardiac arrhythmia including atrial fibrillation, supraventricular tachycardia and extrasystoles -especially if used concomitantly with other p2- agonists Myocardial ischaemia (see section 4.4) |
|
Vascular disorders |
Rare |
Peripheral vasodilatation |
|
Respiratory, thoracic and |
Rare |
Throat irritation |
As with other inhalation therapies, paradoxical bronchospasm may occur immediately after dosing. Salamol CFC-Free Inhaler should be discontinued immediately, the patient reassessed and treated immediately with another presentation or a different fast-acting inhaled bronchodilator.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9. Overdose
Overdosage may result in skeletal muscle tremor, tachycardia, tenseness, headache and peripheral vasodilatation. The preferred antidote for overdosage with salbutamol is a cardioselective B-adrenoceptor blocking agent. Betablocking drugs should be used with caution in patients with a history of bronchospasm, as these drugs are potentially life-threatening. Hypokalaemia may occur following overdose with salbutamol. Serum potassium levels should be monitored.
Hyperglycaemia and agitation have also been reported following overdose with salbutamol.
5 PHARMACOLOGICAL PROPERTIES
5.1
|
mediastinal disorders |
Very rare |
Paradoxical bronchospasm (with an immediate increase in wheezing after dosing) |
|
Gastrointestinal disorders |
Rare |
Mouth irritation, nausea, vomiting, dry mouth, sore mouth |
|
Skin and subcutaneous tissue disorders |
Very rare |
Pruritus |
|
Musculoskeletal and connective |
Uncommon |
Myalgia |
|
tissue disorders |
Rare |
Muscle cramps |
|
Very rare |
Fine tremor (particularly of hands) |
Pharmacodynamic properties
Pharmacotherapeutic group: Selective beta 2 adrenoceptor agonists. ATC Code: R03 AC02
Mechanism of action
At therapeutic doses salbutamol acts on the p2-adrenoceptors of bronchial muscle with little or no action on the p2-adrenoceptors predominantly found in cardiac muscle.
Pharmacodynamic effects
Salbutamol provides short acting (4-6 hours) bronchodilatation with a fast onset (within 5 minutes) in reversible airways obstruction.
Paediatric Population
Paediatric clinical studies conducted at the recommended dose (SB020001. SB030001. SB030002) in patients < 4 years with bronchospasm associated with reversible obstructive airways disease, show that Salbutamol CFC-Free Inhaler has a safety profile comparable to that in children > 4 years, adolescents and adults.
5.2 Pharmacokinetic properties
Salamol CFC-Free Inhaler has been shown to be therapeutically equivalent to salbutamol metered dose inhaler formulated with chlorofluorocarbon (CFC) propellants.
Absorption
Salbutamol is readily absorbed from the gastro-intestinal tract.
Distribution
Salbutamol is subject to first pass metabolism in the liver; about half is excreted in the urine as an inactive sulfate conjugate following oral administration (the rest being unchanged salbutamol). Salbutamol does not appear to be metabolised in the lung, therefore its behaviour following inhalation depends upon the delivery method used which determines the proportion of inhaled salbutamol relative to the proportion inadvertently swallowed.
Elimination
The plasma half-life has been estimated to range from about two to seven hours, the longer values are associated with aerosol inhalation.
5.3 Preclinical safety data
Salamol CFC-Free Inhaler
Toxicological studies in rats and dogs with salbutamol formulated in propellant HFA-134a have shown a comparative safety profile to the current CFC-containing products. The adverse effects noted at high doses were consistent with the known effects of salbutamol inhalation.
Propellant HFA-134a
Toxicological effects of propellant HFA-134a consisted of narcosis and a relatively weak cardiac sensitising potential at very high exposure concentrations only. Safety margins of 2200, 1314 and 381 for mouse, rat and dog with respect to humans have been observed.
Salbutamol
Salbutamol has been used clinically for over 20 years and its safety and efficacy have been proven.
6. PHARMACEUTICAL PARTICULARS
6.1. List of Excipients
Ethanol, anhydrous Norflurane (Propellant HFA-134a)
Salamol CFC-Free Inhaler contains a new propellant (HFA-134a) and does not contain any chlorofluorocarbon (CFC) propellants.
6.2. Incompatibilities
Not applicable.
6.3. Shelf-Life
3 years.
6.4. Special Precautions for Storage
Do not store above 25°C. Do not refrigerate or freeze.
6.5 Nature and contents of container
A pressurised aluminium container with a metering valve. Each pack contains either a single MDI that supplies 200 metered actuations or a twin pack with one MDI and one refill canister, each containing 200 actuations (2 x 200 for Germany only).
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Norton Healthcare Ltd.,
T/A IVAX Pharmaceuticals UK,
Ridings Point,
Whistler Drive,
Castleford,
West Yorkshire,
WF10 5HX,
United Kingdom
8. MARKETING AUTHORISATION NUMBER
PL 00530/0555
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 14th April 2000 Data of last renewal: 14th April 2010
10 DATE OF REVISION OF THE TEXT
26/08/2016