Tobramycin 300 Mg/5 Ml Nebuliser Solution
TEVA UK Ref: 231-30-26117-H LEA TOBRAMYCIN 300mg/5ml NEB SOL TUK <RUN Version: 2 3 June 2016
TEVA UK Ref: 231-30-26117-H LEA TOBRAMYCIN 300mg/5ml NEB SOL TUK <RUN Version: 2 3 June 2016
Tobra
N
myci
ebulis
tobr


1
2 pair of ampoules
I ( >1
of the tubing to the
6
6
1
2
3
4
2
3/4
n 300 mg/5 ml er Solution
amycin
LO
OJ
LU
Q_
Package leaflet: Information for the patient
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to you doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Tobramycin Nebuliser Solution is and what it is used for
2. What you need to know before you use Tobramycin Nebuliser Solution
3. How to use Tobramycin Nebuliser Solution
4. Possible side effects
5. How to store Tobramycin Nebuliser Solution
6. Contents of the pack and other information
OWhat Tobramycin Nebuliser Solution us and what it is used for
Tobramycin Nebuliser Solution contains the active substance tobramycin. This is an aminoglycoside antibiotic.
Tobramycin Nebuliser Solution is used in patients aged six years and older.
Tobramycin Nebuliser Solution is used for treating chest infection in Cystic Fibrosis (CF) caused by a common bacterium, Pseudomonas aeruginosa.It kills the bacterium and helps improve your breathing. As tobramycin is inhaled, rather than taken as a pill, more of the antibiotic gets into your lungs.
What you need to know before you use Tobramycin Nebuliser Solution
Do not use Tobramycin Nebuliser Solution
• if you are allergic to tobramycin or any of the other ingredients of this medicine (listed in section 6) or to any other aminoglycoside antibiotic.
Warnings and precautions
Talk to your doctor or pharmacist before using Tobramycin Nebuliser Solution if any of the following apply to you:
- if you have hearing problems, if you have experienced ringing in your ears, or if you have experienced dizziness, your doctor may test your hearing and balance before starting tobramycin or at any time during tobramycin treatment;
- if you are coughing up blood, your doctor may ask you to stop using tobramycin until there is little or no blood in your sputum;
- if you have had kidney problems, your doctor may check that your kidneys are working properly;
- if you have suffered from muscle weakness - symptoms mostly related to disorders such as myasthenia or Parkinson's disease;
- if you are resistant to antibiotics, you should speak to your doctor.
Take special care with Tobramycin Nebuliser Solution
Inhaled medicines including tobramycin can cause chest tightness. Your doctor will supervise your first dose of Tobramycin Nebuliser Solution and check your lung function before and after dosing. Your doctor may ask you to use a bronchodilator (e.g. salbutamol), if you are not already doing so, before taking Tobramycin Nebuliser Solution.
Children
Do not give this medicine to children less than 6 years old because there is not enough data in this population.
Other medicines and Tobramycin Nebuliser Solution
Tell your doctor or pharmacist if you are taking, have recently taken any other medicines, including medicines obtained without a prescription.
You should not use Tobramycin Nebuliser Solution if you are taking any of the following:
• Diuretics (water tablets) containing furosemide;
• Urea or mannitol, which are used in hospital to treat serious conditions;
• Drugs that may harm your kidneys or hearing could be made worse by Tobramycin Nebuliser Solution.
You may be receiving injections of tobramycin or other aminoglycosides as well as using inhaled tobramycin. Such injections, which may increase the very low levels of aminoglycosides caused by inhaled tobramycin, are not recommended when the following medicines are also being taken:
• Amphotericin B, cefalotin, ciclosporin, tacrolimus, polymixins
• Platinum compounds (e.g., carboplatin and cisplatin)
• Anticholinesterases (e.g., neostigmine and pyridostigmine), botulinum toxin.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
The possible side effects of inhaling tobramycin in pregnant women are not known. However, higher blood levels of tobramycin and similar medicines, which can occur when they are given by injection, can harm an unborn baby (e.g., by causing deafness).
Breast feeding
It is unknown whether inhaled tobramycin can be detected in breast-milk at the recommended dose.
Fertility
Possible effects on fertility are unlikely, but cannot be excluded.
Driving and using machines
This medicine is not expected to affect your ability to drive and use machines.
©How to use Tobramycin Nebuliser Solution
For inhalation use.
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is two ampoules per day for 28 days. The usual dose is the same for all persons aged 6 years and older. Inhale the contents of one ampoule in the morning and one in the evening. There should be a 12-hour gap between the doses.
You then have 28 days without taking your medicine before starting another 28-day treatment course again.
It is important that you keep using the product twice each day during your 28 days on treatment and that you keep to the 28-day on/28-day off cycle.
Tobramycin Nebuliser Solution should be used with a clean, dry PARI LC PLUS reusable nebuliser and a suitable compressor. Ask your doctor or physiotherpist for advice on which compressor to use. Your doctor or physiotherapist can advise you on the proper use of your medicine and the equipment you need. You may need different nebulisers for other inhaled medicines such as dornase alfa, which can be used to improve sputum clearance in CF.
Before you start to use Tobramycin Nebuliser Solution, make sure you have the following pieces of equipment:
• Tobramycin ampoule
• PARI LC PLUS reusable nebuliser
• Suitable compressor
• Tubing to connect the nebuliser and compressor
• Clean paper or cloth towels
• Nose clips (if required)
You should check that your nebuliser and compressor are working properly according to the manufacturer's instructions before you start to use your medicine.
Preparing your medicine for inhalation
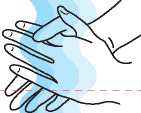
1. Wash your hands
thoroughly with soap and water.
2. Each foil pouch contains 4 ampoules. Cut or tear open the pouch, remove an ampoule and put the foil pouch in the refrigerator.
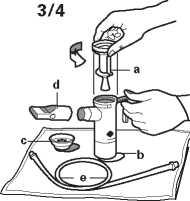
3. Lay out the pieces of your PARI LC PLUS nebuliser on a clean, dry paper or cloth towel.
You should have the following pieces:
a. Nebuliser top
b. Nebuliser bottom
c. Inspiratory valve cap
d. Mouthpiece with valve
e. Tubing.
4. Remove the nebuliser top from the nebuliser bottom by twisting the top anticlockwise and then lifting it. Place the top on the towel and stand the nebuliser bottom upright on the towel.
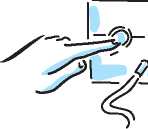
5. Connect one end compressor air outlet. Make sure that the tubing fits snugly and plug the compressor into the
electrical outlet.
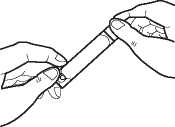
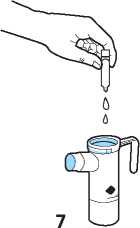
Open the ampoule by holding the bottom tab with one hand and twisting off the top with your
other hand. Be careful not to squeeze the ampoule until you are ready to empty it into the nebuliser bottom.
7 When you are ready, squeeze all of the contents of the ampoule into the nebuliser bottom.
8. Replace the nebuliser top. Turn the top clockwise until it is securely fastened to the nebuliser bottom.
9. Attach the mouthpiece to the nebuliser outlet.
Firmly push the inspiratory valve cap in place on the nebuliser top. The inspiratory valve cap must fit snugly (check with your PARI LC PLUS nebuliser leaflet).
10. Connect the free end of the tubing to the air intake on the bottom of the nebuliser, making sure you keep the nebuliser upright. Press the tubing on the air intake firmly.
Taking your medicine
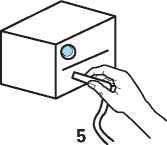
Turn on the compressor.
1
Make sure there is a steady mist coming from the
mouthpiece. If there is no mist, check that all the tubing connections and that the compressor is working correctly.
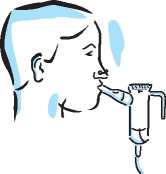
Sit or stand in an upright position so that you can breathe normally.
Place the mouthpiece between your teeth and on top of your tongue. Breathe through your mouth (you may find that nose clips help). Try not to block the airflow with your tongue.
5 Continue until all of the Tobramycin Nebuliser Solution is gone and there is no longer any mist being produced.


6 Please remember to clean and disinfect your nebuliser after treatment. You should never use a dirty or clogged nebuliser. You should not share your nebuliser with other people.
It should take about 15 minutes to take all of the medicine. You may hear a spluttering sound when the nebuliser cup is empty. If you are interrupted or need to cough or rest during the treatment, turn off the compressor to save your medicine. When you are ready to restart your treamtent, turn the compressor on again.
If you are taking several different treatments for CF, you should take them in the following order:
• bronchodilator (e.g., salbutamol)
• chest physiotherapy
• other inhaled medicines
• and then Tobramycin Nebuliser Solution.
If you are not sure, check the order with your doctor.
If you use more Tobramycin Nebuliser Solution than you should
If you inhale too much tobramycin you may get a very hoarse voice. Make sure you tell your doctor as soon as possible.
If you accidentally swallow Tobramycin Nebuliser Solution, don't worry, but tell your doctor as soon as possible.
If you forget to use Tobramycin Nebuliser Solution
Do not take a double dose to make up for a forgotten dose.
You must not take two doses within six hours. Take the dose immediately if there are at least six hours to your next dose or miss out this dose if your next dose is due in less than six hours.
If you stop using Tobramycin Nebuliser Solution
You should not stop using Tobramycin Nebuliser Solution until you have completed the course of treatment or told to do so by your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects can be serious
• Chest tightness with difficulty breathing (rare)
• Allergic and hypersensitivity reactions including hives and itching (very rare)
If any of these serious side effects happen to you, stop taking Tobramycin Nebuliser Solution and tell your doctor immediately.
The following side effects have been reported at the approximate frequencies shown:
Uncommon (may affect up to 1 in 100 people):
• Voice alteration (hoarseness)
• Increased cough
• Shortness of breath
• Sore throat
Rare (may affect up to 1 in 1,000 people):
• Chest pain
• General pain
• Laryngitis (voice alteration with sore throat and difficulty swallowing)
• Ringing in the ears
• Mouth ulceration
• Rash
• Weakness
• Fever
• Headache
• Feeling sick (nausea)
• Being sick (vomiting)
• Loss of appetite
• Dizziness
• Increased sputum quantity
• Coughing up blood
• Loss of voice
• Nose bleeds
• Runny nose
• Taste disturbances
• Asthma
• Back pain
• Patients who have received Tobramycin Nebuliser Solution at the same time as, or following repeated doses of injected tobramycin or related drugs, may develop hearing loss.
• Swelling of the lymph glands
• Sleepiness
• Hyperventilating
• Sinusitis
• General feeling of being unwell
People with cystic fibrosis experience many symptoms of the disease. These may still occur while taking Tobramycin Nebuliser Solution but should not be any more frequent or seem worse than before. Patients have commonly reported symptoms such as sputum discolouration, chest infection, muscle pain, nasal polyps and ear infections.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
How to store Tobramycin Nebuliser Solution
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer packaging, pouch and ampoule after EXP The expiry date refers to the last day of that month.
Store in a refrigerator (2 - 8°C). Do not freeze. You can store the foil pouches (opened or unopened) at room temperature (do not store above 25°C) for up to 28 days. Store in the original package in order to protect from light. Never store an opened ampoule. Once opened the ampoule should be used immediately.
Do not use this medicine if you notice any visible signs of deterioration (cloudiness or bits in the solution). Tobramycin solution may be slightly yellow and some variability in colour may be observed; this does not indicate loss of activity providing the solution has been stored as recommended.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
©Contents of the pack and other information
What Tobramycin Nebuliser Solution contains
• The active substance is tobramycin. Each 5 ml single dose ampoule contains 300 mg of tobramycin..
• The other ingredients are: sodium chloride, Water for injections, sulfuric acid (for pH-adjustment) and sodium hydroxide (for pH-adjustment).
What Tobramycin Nebuliser Solution looks like and contents of the pack
Tobramycin Nebuliser Solution is a clear to slightly yellow solution.
Tobramycin Nebuliser Solution is supplied in 5 ml, single dose ampoules.
4 ampoules are packed and sealed in a foil pouch.
Each carton comprises 14 (56 ampoules), 28 (T12 ampoules) or 42 (168 ampoules) foil pouches, which is enough to last one, two or three cycles of treatment, respectively.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Teva UK Limited, Eastbourne,
BN22 9AG, UK
Manufacturer:
Norton Healthcare Limited T/A Ivax Pharmaceuticals UK,
Preston Brook,
Runcorn,
Cheshire,
WA7 3FA,
United Kingdom
This leaflet was last revised in 05/2016
PL 00289/1437
TEUZD
Very rare (may affect up to 1 in 10,000 people):
• Abdominal pain
• Ear pain
• Diarrhoea
26117-H
• Fungal infections (e.g., thrush)
This is a representation of an electronic record that was signed electronically and this
PAGE IS THE MANIFESTATION OF THE ELECTRONIC SIGNATURE
Teva Pharmaceuticals Europe B.V
1.3.2 mockup-pil-uk-pl00289-1437-tobramycin-300mg-5ml-nebuliser-solution
Approvals
|
Signed by |
Meaning of Signature |
Server Date |
|
Michael Silvester |
Regulatory Affairs Approval |
08-Jun-2016 02:17:30 PM |
REG0093792 Version 6.2 Approved Page 3 of 3